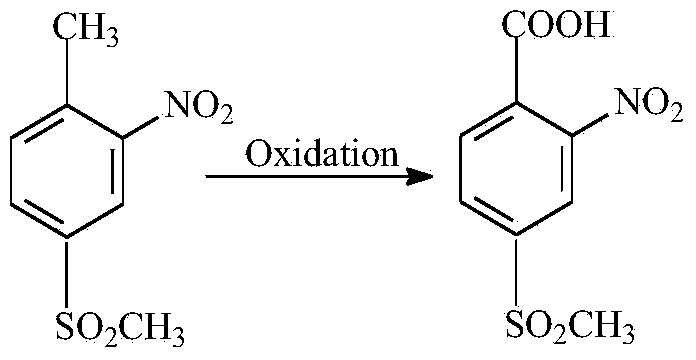
Preparation method of 2-nitro-4-methylsulphonylbenzoic acid
A technology of methylsulfonyl benzoic acid and methylsulfonyl toluene is applied in the field of catalytic oxidation synthesis technology of 2-nitro-4-methylsulfonyl benzoic acid, and can solve the problem that the degree of oxidation reaction is difficult to control, the oxidizing ability is limited, and the Difficulty in methyl oxidation and other problems, to achieve the effect of reducing waste water pollution, high conversion rate and selectivity, and eliminating waste gas pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] The experiment was carried out in a stirred reactor with a volume of 1000ml, glacial acetic acid 800ml, water 50g, 2-nitro-4-thiamphenicol toluene 80g, phosphomolybdovanadate 6g, manganese sulfate 9.5g, potassium iodide 0.6g were added, and the reaction The temperature is 180°C, the pressure is 40atm, and the air flow rate is 500ml / min. After the reaction starts, liquid samples are taken every 0.5h and analyzed by liquid chromatography. After 4h of reaction, the conversion rate of 2-nitro-4-thiamphenicol toluene reaches 95%. , The yield of 2-nitro-4-thiamphenicol benzoic acid reached 91%.
[0055] The reaction solution was cooled to 5°C, and the precipitate was separated from the reaction solution by filtration.
[0056] Gained solid precipitation is fully dissolved with the sodium hydroxide solution of 200ml 15% (mass), filters insoluble solid impurity, and the filtrate of filtering gained is adjusted to pH1.0 with sulfuric acid again, and crystallization precipitates ...
Embodiment 2
[0059] The experiment was carried out in a reactor with a volume of 1000ml. The gas-liquid two-phase flowed through the reactor continuously from bottom to top. The flow rate of the liquid was 5ml / min, and the flow rate of the air was 500ml / min. In the liquid feed, 2-nitro- 10% (mass) of 4-thiamphenicol toluene, 10% (mass) of water, 0.70% (mass) of phosphomolybdovanadic acid, 0.30% (mass) of manganese, 0.05% (mass) of iodine, the rest are acetic acid, and the reaction temperature is 210 ℃, pressure 30atm, the outlet liquid of the reactor was analyzed by liquid chromatography, the conversion rate of 2-nitro-4-thiamphenicol toluene reached 96%, and the yield of 2-nitro-4-thiamphenicol benzoic acid reached 91%.
Embodiment 3
[0061] The experiment was carried out in a stirred reactor with a volume of 1000ml, adding 800ml of glacial acetic acid, 80g of water, 80g of 2-nitro-4-thiamphenicol toluene, 4g of phosphotungstic acid, 2g of cobalt acetate, 4g of manganese acetate, tetrabromoethyl Alkane 0.5g, reaction temperature 210 ℃, pressure 30atm, air flow rate is 500ml / min, after the reaction begins, take liquid sample every 0.5h and use liquid chromatography analysis, after reaction 4h, 2-nitro-4-thiamphenicol toluene The conversion rate reaches 98%, and the yield of 2-nitro-4-thiamphenicol benzoic acid reaches 91%.
[0062] The reaction solution was cooled to 5°C, and the precipitate was separated from the reaction solution by filtration.
[0063] The resulting solid precipitate was purified by the method described in Example 1 to obtain 80.3 g of pale yellow 2-nitro-4-thiamphenicol benzoic acid.
[0064] Add 50ml of glacial acetic acid to the resulting filtrate, then add 80g of 2-nitro-4-thiampheni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com