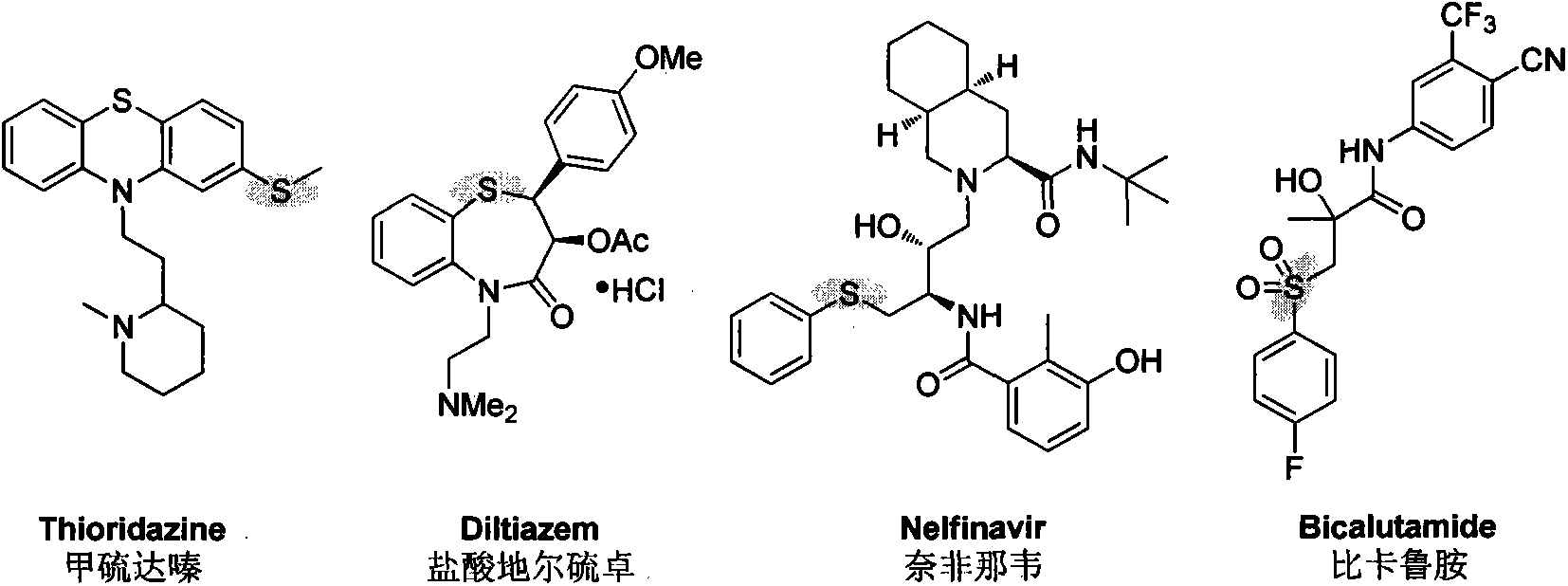
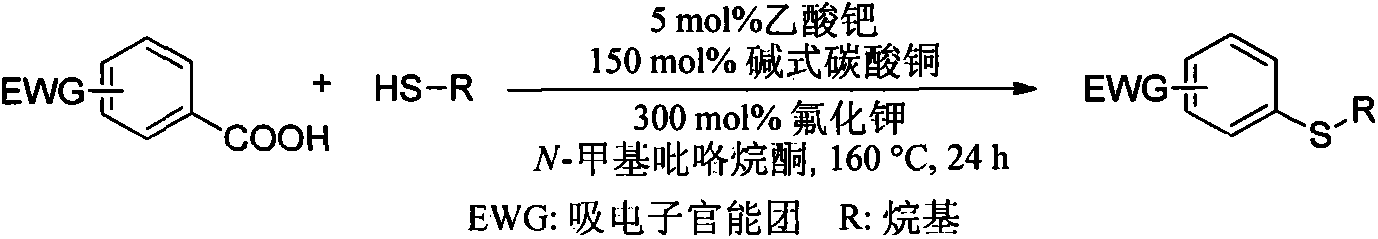
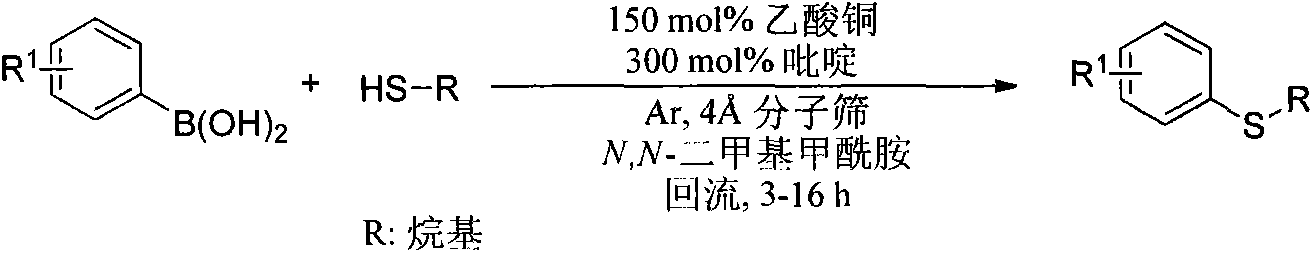
Synthetic method of aryl-alkyl thioether compound
An aryl alkyl sulfide and a synthesis method technology, applied in the preparation of sulfide, organic chemistry and other directions, can solve the problems of limited application and high cost of aryl boronic acid substrates, achieve simple operation, no need for inert gas protection, and reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 takes sodium p-toluene sulfinate as raw material to synthesize 3-(p-tolylmercapto) methyl propionate:
[0021]
[0022] In a 10mL microwave reaction flask equipped with a magnet, add sodium p-toluenesulfinate (substrate 1a, 66.8mg, 0.36mmol), palladium chloride (10.6mg, 0.06mmol), silver carbonate (168.0mg, 0.6 mmol), solvent N,N-dimethylformamide / dimethyl sulfoxide (volume ratio 19 / 1, 2 mL), methyl 3-mercaptopropionate (substrate 2a, 38.04 μL, 0.3 mmol). Cover with a lid and stir at room temperature for 5 min. Then, react at 120° C. for 10 min under the heating power of 40 watts in a microwave reactor. TLC monitored the completion of the reaction. After the system was cooled to room temperature, it was filtered through a celite column and washed with dichloromethane. A small amount of silica gel was added, spin-dried, and flash column chromatography gave 15.8 mg of a colorless transparent liquid with a yield of 25%.
[0023] R f =0.44(V 正己烷 / V 乙酸...
Embodiment 2
[0024] Embodiment 2 takes sodium p-toluene sulfinate as raw material to synthesize 3-(p-tolylmercapto) methyl propionate:
[0025]
[0026] Operation method is the same as embodiment 1. The difference is that tetrahydrofuran (2 mL) was used instead of N,N-dimethylformamide and dimethyl sulfoxide as solvent, and 120W was used instead of 40W as microwave heating power. 44.1 mg of a colorless transparent liquid was obtained with a yield of 70%. Its R f , 1 H NMR, 13 C NMR, MS and other analytical data are the same as in Example 1.
Embodiment 3
[0028] Taking sodium p-toluene sulfinate as raw material to synthesize 3-(p-tolylmercapto) methyl propionate:
[0029]
[0030] Operation method is the same as embodiment 1. The difference is that 1,4-dioxane (2 mL) was used instead of N,A-dimethylformamide / dimethyl sulfoxide as solvent, and 180 W was used instead of 40 W as microwave heating power. 42.8 mg of a colorless transparent liquid was obtained with a yield of 68%. Its R f , 1 H NMR, 13 C NMR, MS and other analytical data are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com