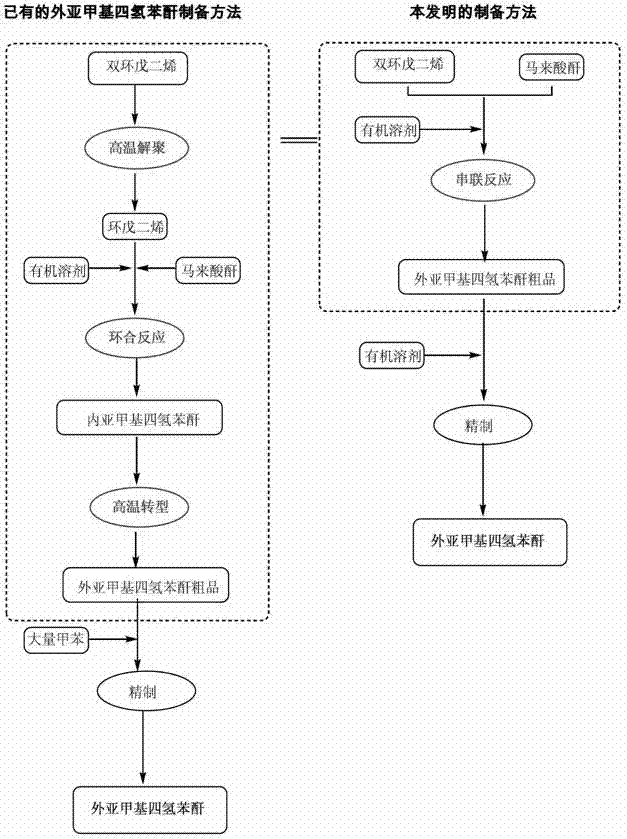
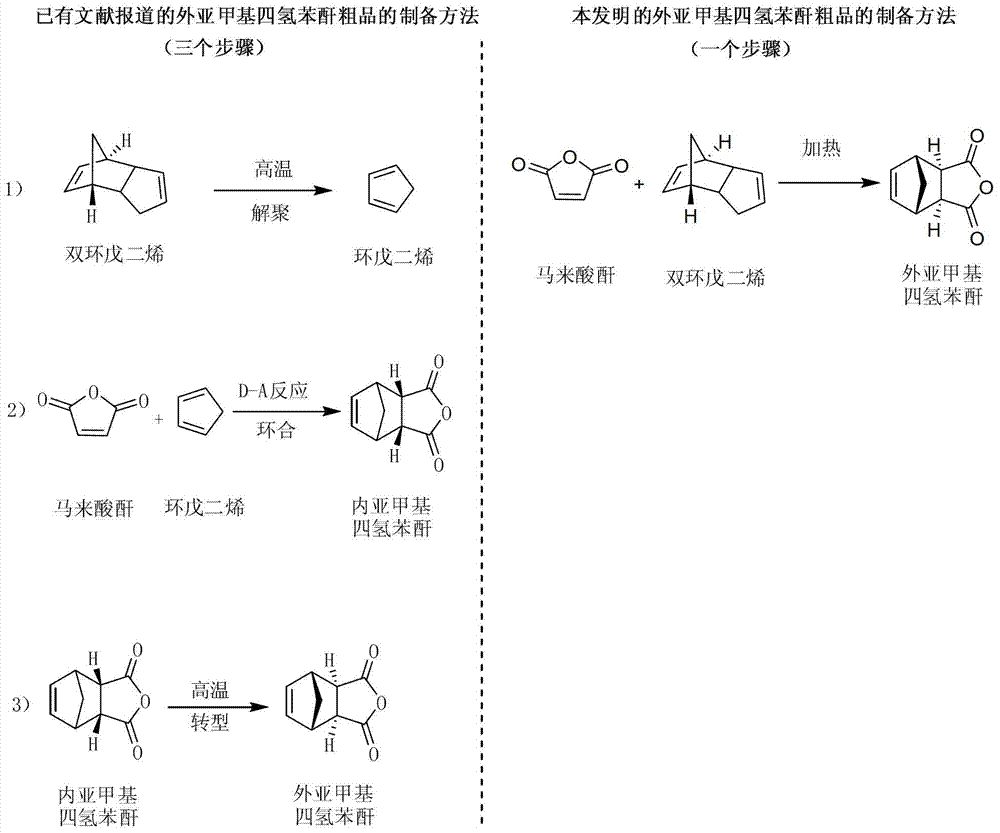
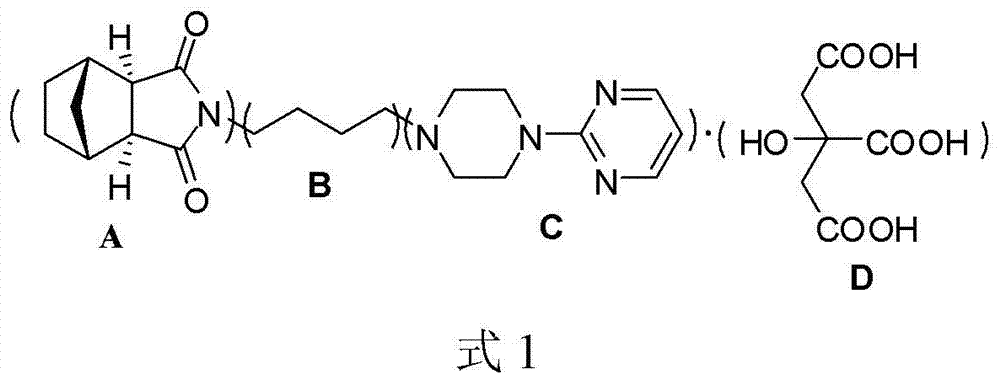
Preparation and purification method of exomethylenetetrahydrophthalic anhydride and its application in the preparation of tandospirone
A technology of exomethylenetetrahydrophthalic anhydride and methylenetetrahydrophthalic anhydride, which is applied in the field of compound synthesis, can solve the problems of low refining yield, environmental threat, and prone to multimerization, and achieve high crude product quality and solve toxicity big effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1 Preparation of exo-methylene tetrahydrophthalic anhydride
[0062] Weigh 98 g of maleic anhydride and 26 g of dicyclopentadiene, place them in a reaction kettle, add 100 mL of dichloromethane, seal, stir, and heat up to 150 ° C. After the reaction is complete, the resulting reaction solution is cooled and crystallized. , separated and dried to obtain 47.5 g of crude external methylene tetrahydrophthalic anhydride (yield 72.35%).
Embodiment 2
[0065] Example 2 Preparation of exo-methylene tetrahydrophthalic anhydride
[0066] Weigh 98 g of maleic anhydride and 528 g of dicyclopentadiene, place them in a reaction kettle, add 2000 mL of dichloromethane, seal, stir, and heat up to 220 ° C. After the reaction is complete, the resulting reaction solution is cooled and crystallized. , separated and dried to obtain 144.7g of crude external methylenetetrahydrophthalic anhydride (yield 88.26%).
[0067] The retention time of the main peak was consistent with that of the reference substance.
[0068] Chromatographic purity: 67.33%.
Embodiment 3
[0069] Example 3 Preparation of exo-methylene tetrahydrophthalic anhydride
[0070] Weigh 98 g of maleic anhydride and 793 g of dicyclopentadiene, place them in a reactor, add 6000 mL of ethanol, seal, stir, heat up to 120 ° C, and after the reaction is complete, cool the resulting reaction solution, crystallize, and separate , and dried to obtain 145.2 g of crude external methylene tetrahydrophthalic anhydride (yield 88.51%).
[0071] The retention time of the main peak was consistent with that of the reference substance.
[0072] Chromatographic purity: 67.54%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com