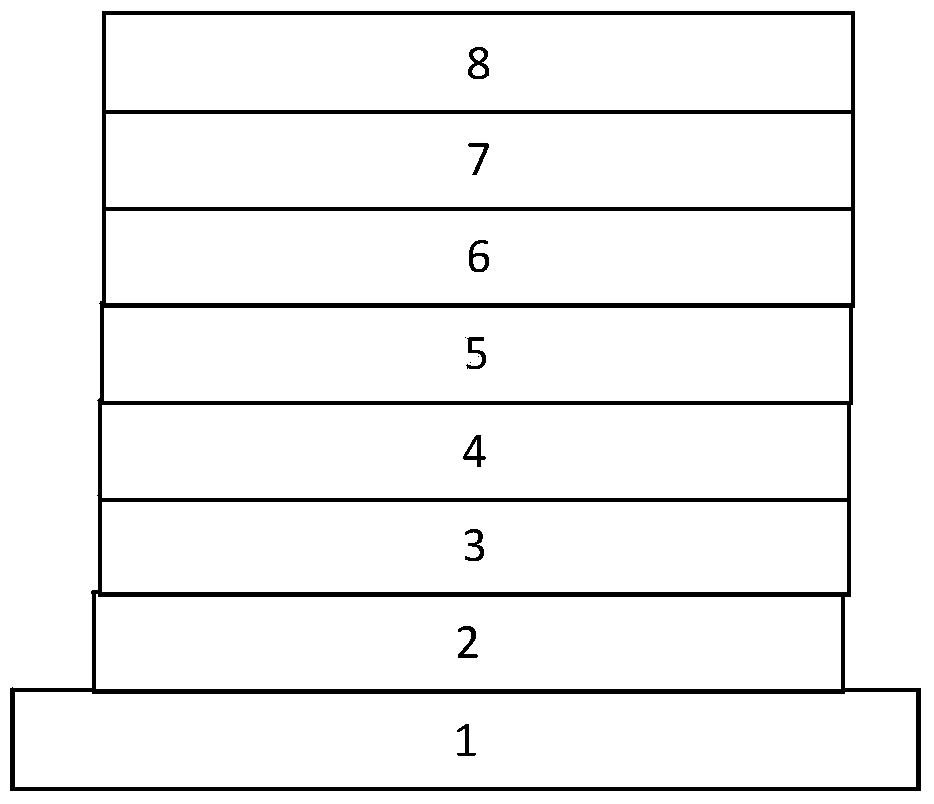
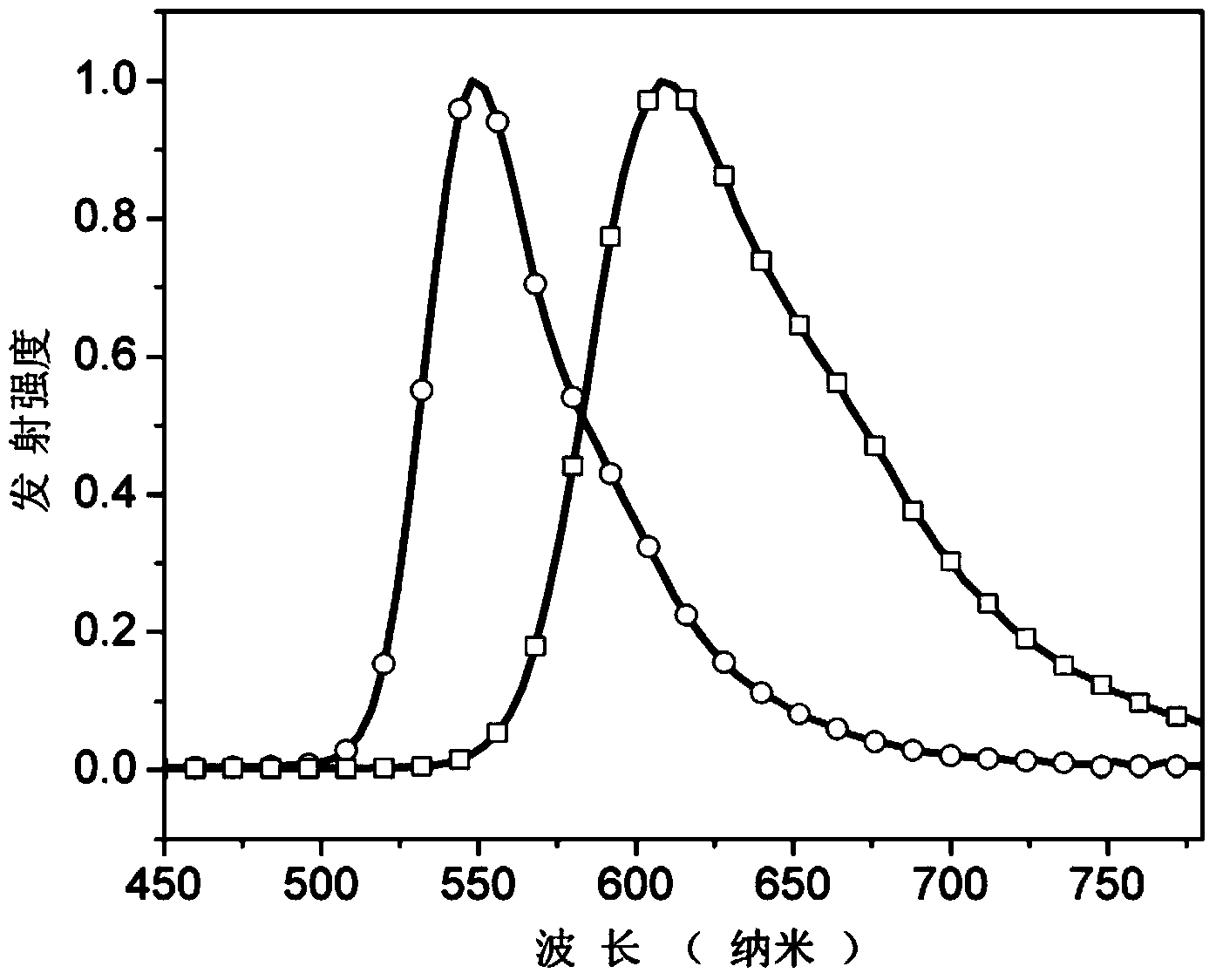
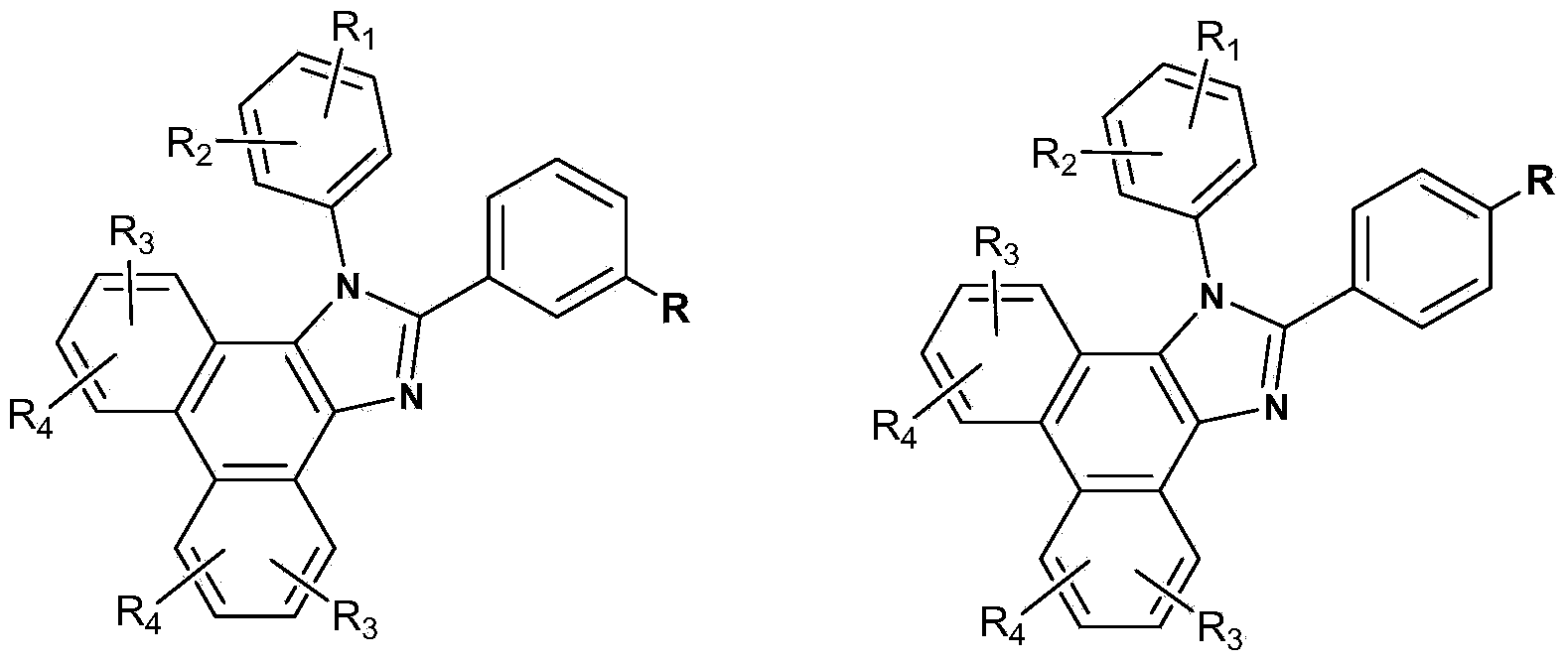
Main material based on phenanthrene and imidazole derivative and electroluminescent device
An electroluminescent device, phenanthroimidazole technology, applied in the field of electroluminescent devices, can solve problems such as stagnation, no practical use of the process, and difficult growth of single crystals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of compound 1:
[0023] Add phenanthrenequinone (830mg, 4mmol), m-phthalaldehyde (528mg, 4mmol), aniline (1.48g, 16mmol), ammonium acetate (1.6g, 20mmol), and acetic acid (40ml) into a three-necked flask, under N2 protection, in The oil bath was heated to reflux at 123°C for 12h. Stop the reaction, pour the reaction mixture into distilled water, stir and filter, the obtained gray filter cake is washed with water, glacial acetic acid, and ethanol in sequence, and after drying, the monoaldehyde product is obtained. The intermediate (339mg, 1mmol), 1,10-phenanthrene Add roline-5,6-dione (208mg, 1mmol), ammonium acetate (308mg, 4mmol), aniline (465mg, 5mmol), and acetic acid (20ml) into a three-necked flask, heat and reflux in an oil bath at 123°C for 12 hours, stop The reaction was poured into water, and the filter cake was washed with water and ethanol, and vacuum sublimated to obtain a yellow product (312 mg, yield 47%). The molecular ion ma...
Embodiment 2
[0024] Embodiment 2: the synthesis of compound 2:
[0025] Add phenanthrenequinone (4.16g, 20mmol), p-bromobenzaldehyde (4.07g, 22mmol), ammonium acetate (6.16g, 80mmol), aniline (9.3g, 100mmol) and 160ml of glacial acetic acid into a 250ml two-necked bottle. N 2 Under protection, the mixture was heated and refluxed for 12 hours, the reaction was stopped, suction filtered, and the filter cake was washed with water and methanol in sequence to obtain the meta-bromo-substituted phenanthroimidazole intermediate (6.81g, yield 76%), and the product was directly dried to make Next reaction. The meta-bromo-substituted phenanthroimidazole (0.90 g, 2 mmol) obtained above was dissolved in 30 ml of freshly distilled THF mixture, and cooled to -78°C with a liquid nitrogen / acetone bath. The deoxygenation operation was repeated several times by evacuating nitrogen, and then adding n-butyllithium (0.88ml, 2.5M n-hexane solution) dropwise. The solution was kept at -78°C and stirred for thre...
Embodiment 3
[0026] Embodiment 3: the synthesis of compound 3:
[0027] Cool the above-mentioned 30ml freshly distilled THF solution with meta-bromine-substituted phenanthroimidazole (2.04g, 4.5mmol) to -78°C with a liquid nitrogen / acetone bath, vacuumize and ventilate nitrogen repeatedly to remove oxygen and then drop by drop Add n-butyllithium (2.5M n-hexane solution, 2ml), stir at this temperature for 3 hours, then add phenyl phosphorus dichloride (0.27g, 1.5mmol) dropwise, then slowly return to room temperature and continue stirring 12 hours. Add 30 ml of methanol to the reaction and extract with dichloromethane, combine the organic phases with anhydrous MgSO 4 Dry and spin the solvent to purify by column chromatography (silica gel, dichloromethane / ethyl acetate volume ratio=20 / 1). The resulting crude product was dissolved in 10ml of dichloromethane, added aqueous hydrogen peroxide solution (30%, 0.4ml, 3mmol), and stirred at room temperature for 12 hours. The reaction was stopped, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com