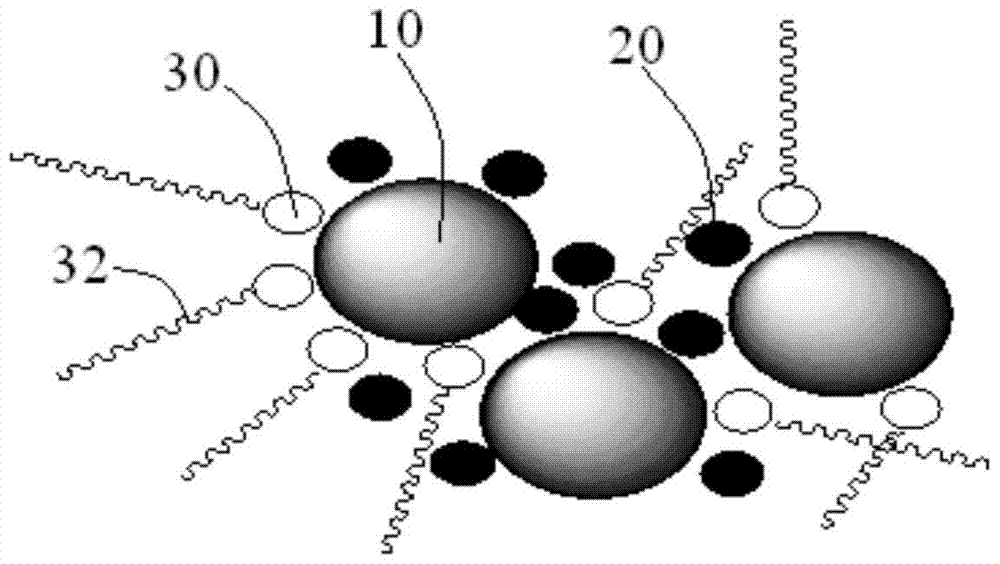
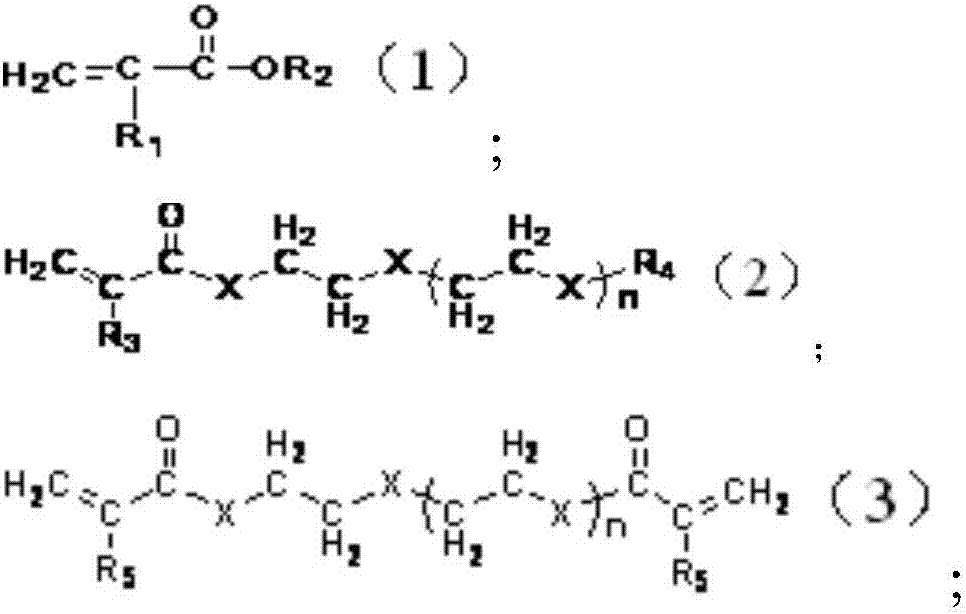
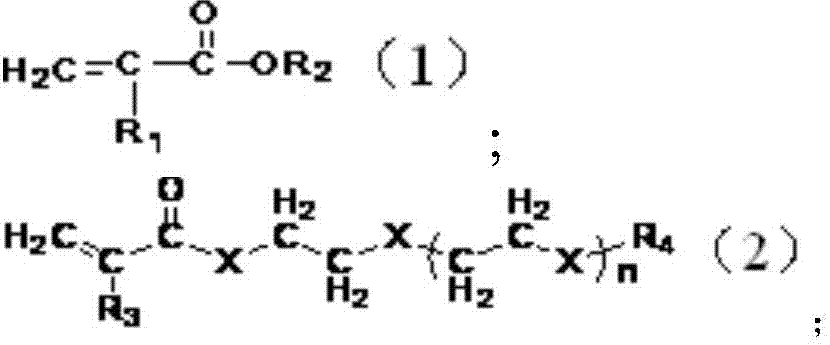Lithium-ion secondary battery and negative electrode sheet thereof
A technology of secondary battery and negative electrode sheet, applied in secondary battery, battery electrode, non-aqueous electrolyte battery electrode, etc., can solve the problems of poor battery cycle performance, battery polarization phenomenon, large irreversible capacity loss, etc. The effect of improving storage performance and charge-discharge rate performance, improving first-time charge-discharge efficiency, and improving ion-conducting performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of adhesive: After the air in the three-neck glass flask with an inner volume of 2L was replaced with nitrogen, butyl acrylate 364g, polyethylene glycol methyl ether acrylate (weight average molecular weight 480) 150g, polyethylene glycol Diacrylate (weight average molecular weight 600) 2g is added in the flask; Dissolve 0.2g of azobisisobutyronitrile (AIBN) in 900g of deionized water, add in the above-mentioned flask, detect the content of acrylic acid by gas chromatography simultaneously; Polymerization reaction After 20 hours at 50°C, the polymer was separated by decantation, vacuum-dried at normal temperature for 24 hours, and then vacuum-dried at 100°C for 10 hours to obtain 280 g of a polymer. The number average molecular weight of the polymer was measured to be 150,000, and the dispersion coefficient was 1.6.
[0035] Preparation of negative electrode sheet: Dissolve the polymer prepared above in deionized water, add carbon-coated Si powder used as ne...
Embodiment 2
[0038] Preparation of adhesive: After replacing the air in a three-necked glass flask with an inner volume of 2L with nitrogen, 56g of methyl acrylate, 108g of polyethylene glycol methyl ether methacrylate (weight average molecular weight 480), polyethylene glycol Glycol dimethacrylate (weight-average molecular weight 600) 36g is added in the flask; Benzoyl peroxide (BPO) 20g is dissolved in DMF (1000g), adds in the above-mentioned flask, detects methyl acrylate by gas chromatography simultaneously Content: After the polymerization reaction was carried out at 100° C. for 16 hours, the polymer was separated by decantation, vacuum-dried at room temperature for 24 hours, and then vacuum-dried at 100° C. for 10 hours to obtain 196 grams of polymer. The number average molecular weight of the polymer was measured to be 350,000, and the dispersion coefficient was 1.5.
[0039] Preparation of negative electrode sheet: Dissolve the polymer prepared above in deionized water, add carbon-...
Embodiment 3
[0042] Preparation of adhesive: After replacing the air in a three-necked glass flask with an inner volume of 2L with nitrogen, 67g of methyl methacrylate and 37g of polyethylenediamine dimethylacrylamide (weight average molecular weight 500) were added to the flask Medium; Dissolve 1 g of azobisisoheptanonitrile (ABVN) in 1000 g of DMF, add it to the above-mentioned flask, and detect the content of methyl methacrylate by gas chromatography; after the polymerization reaction is carried out at 70 ° C for 40 hours, the The polymer was isolated, vacuum-dried at normal temperature for 24 hours, and then vacuum-dried at 80° C. for 10 hours to obtain 82 g of polymer. The number average molecular weight of the polymer was measured to be 500,000, and the dispersion coefficient was 1.7.
[0043] Preparation of negative electrode sheet: Dissolve the polymer prepared above in deionized water, add carbon-coated Si powder used as negative electrode active material and acetylene black used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com