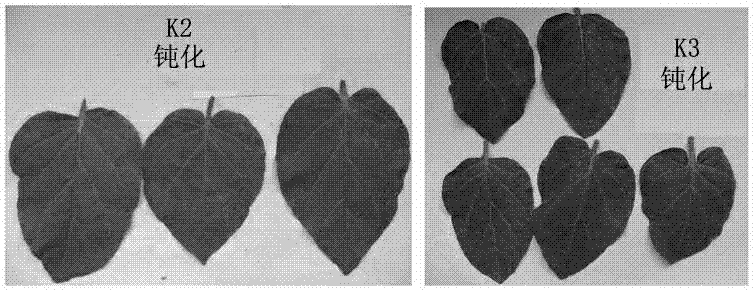
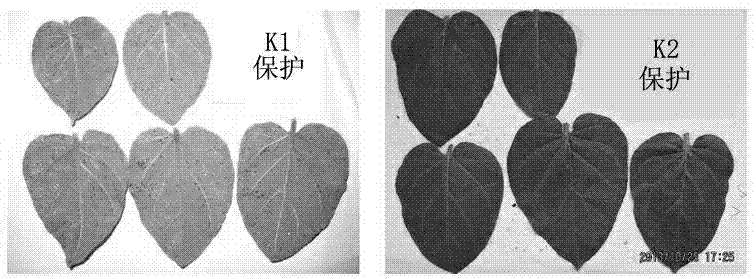
1-substituted-5-amino-4-pyrazol di-1,3,4-diazole sulfur ether or 1,3,4-diazole sulfone derivatives and application thereof
A technology of oxadiazole sulfide and oxadiazole sulfone, which is applied in the field of prevention and control of agricultural plant virus diseases, can solve economic losses, threaten the continuous, stable, and healthy development of the tobacco industry, and reduce local fiscal revenue, tobacco output and quality, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Preparation of 1-(3-chloropyridin-2-yl)-4-((5-methylthio)-1,3,4-thiadiazolyl)-5-amino-1H-pyrazole (A1)
[0039] 1) Preparation of 1-(3-chloropyridin-2-yl)-5-amino-1H-4-pyrazole hydrazide
[0040]
[0041]In a 250 mL three-neck flask equipped with a condenser tube and a thermometer, add ethyl ethoxymethylene acetate (62.1 mmol), 3-chloro-2-pyridinehydrazine (59.1 mmol), and ethanol (50 mL) in sequence, and heat to 80°C, reacted for 24 h, TLC detected the end of the reaction, lowered to room temperature, distilled off the solvent under reduced pressure, the remaining solid was directly dissolved in absolute ethanol (200 mL) without purification, and 80% hydrazine hydrate solution (100 mmol) was slowly added to the reaction solution, heated to reflux for 2 hours, TLC detected that the reaction was complete, the system was lowered to room temperature, filtered with suction, the solid was washed with ethanol, and dried to obtain the product, a white solid, wi...
Embodiment 2
[0048] Example 2: 1-(3-chloropyridin-2-yl)-4-((5-ethylthio)-1,3,4-oxadiazolyl)-5-amino-1H-pyrazole ( A22 ) preparation
[0049] 1) Preparation of 1-(3-chloropyridin-2-yl)-5-amino-1H-4-pyrazole hydrazide
[0050] Wherein the preparation and embodiment of 1-(3-chloropyridin-2-yl)-5-amino-1H-4-pyrazole hydrazide 1 exactly the same in .
[0051] 2) Preparation of 5-(5-amino-1-(3-chloropyridyl-2-yl)-1H-pyrazol-4-yl)-1,3,4-oxadiazole-2-thiol
[0052]
[0053] In a 250 mL three-neck flask with a condenser tube and a thermometer, add 1-(3-chloropyridin-2-yl)-5-amino-1H-4-pyrazole hydrazide (13.1 mmol), potassium hydroxide ( 13.1mol), carbon disulfide (26.1mmol) and ethanol (100 mL), stirred at room temperature for 24 hours, TCL detected the end of the reaction, removed the solvent, without further purification, directly added to 50% potassium hydroxide aqueous solution (100 mL), 50 o After stirring at C for 24 hours, the reaction was detected by TLC, suction filtered, the fil...
Embodiment 3
[0057] Example 3: 1-(3-chloropyridin-2-yl)-4-((5-ethanesulfonyl)-1,3,4-thiadiazolyl)-5-amino-1H-pyrazole ( B2 ) preparation
[0058]
[0059] In a 25 mL three-necked flask with a condenser tube and a thermometer, add 1-(3-chloropyridin-2-yl)-4-((5-ethylthio)-1,3,4-thiadiazolyl )-5-amino-1H-pyrazole (615.8 μmol), sodium tungstate (307.9 μmol), acetic acid (3 mL) and 30% H 2 O 2 (3.7 mmol), react at room temperature for 24 hours, TLC detects the end of the reaction, adjust the pH value to 7~8, filter with suction, and purify the crude product by column chromatography (dichloromethane:ethyl acetate=10:1), the target compound B2. White solid, yield 76.3%. 1 H NMR (DMSO-d 6 ) δ: 8.60 (d, 1H, J = 3.5 Hz, pyridine H) 8.27(d, 1H, J = 8.1 Hz, pyridine H), 8.12 (s, 1H, pyrazole H), 7.66 (q, 1H, J = 8.1 Hz, pyridine H), 7.00 (s, 2H, NH 2 ), 3.71 (q, 2H, J = 7.5 Hz, CH 2 ), 1.30 (t, 3H, J = 6.9 Hz, CH 3 ) ; IR (KBr): ν 3404, 3288, 3074, 2974, 2935, 1602, 1550, 14...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com