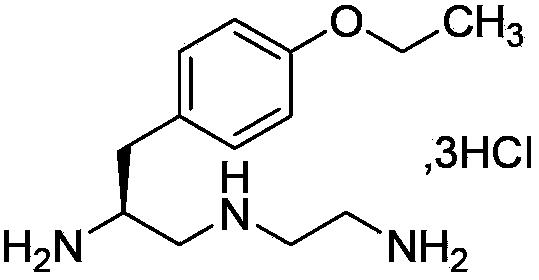
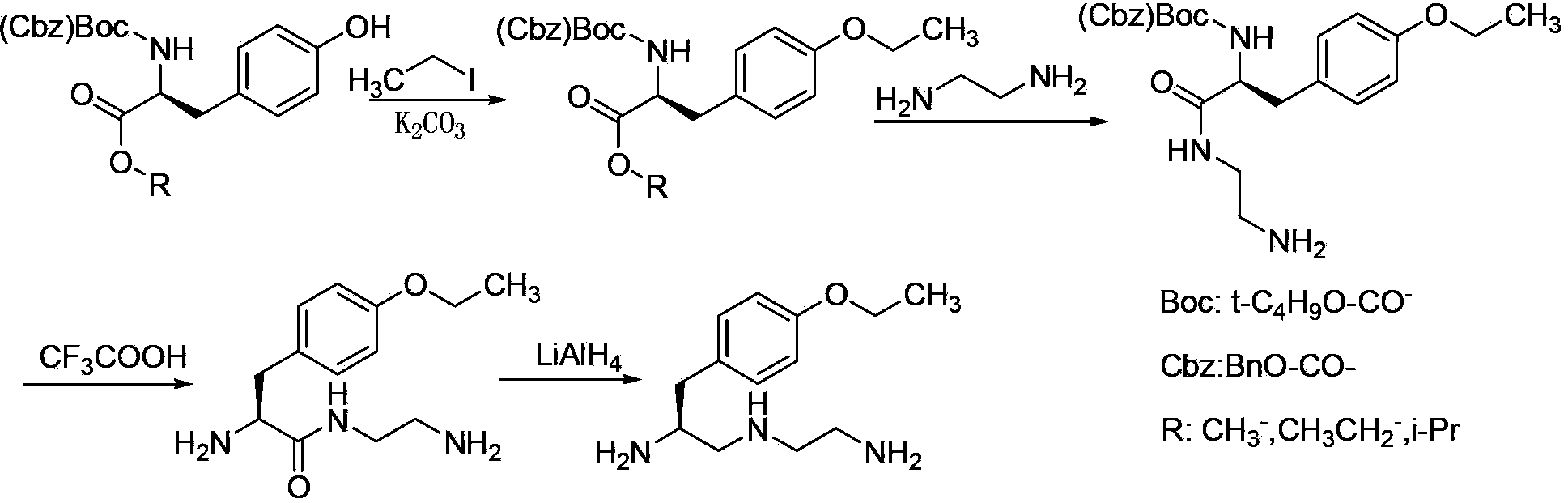
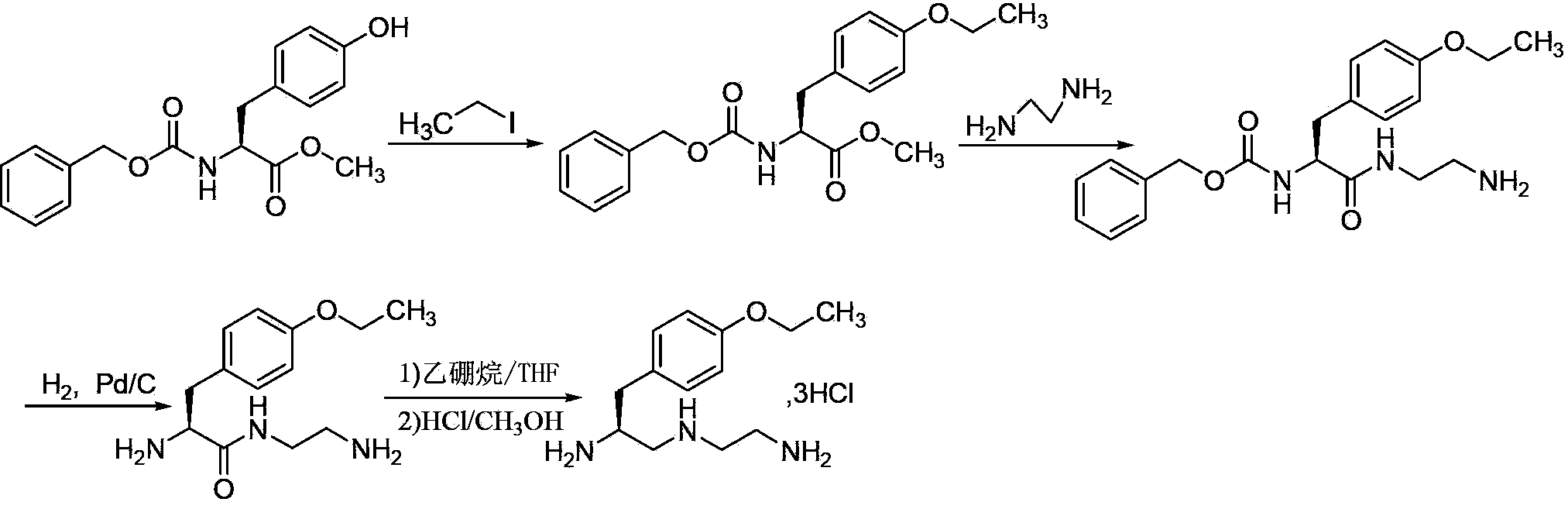
Preparation method of S-1-(4-ethyoxylbenzyl)-3-azapentane-1,5-diaminetrihydrochloride
A technology of diamine trihydrochloride and ethoxybenzyl is applied in the preparation of amino hydroxy compounds, the preparation of organic compounds, chemical instruments and methods, etc. Difficulty in post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] A preparation method of S-1-(4-ethoxybenzyl)-3-azopentane-1,5 diamine trihydrochloride, comprising the following steps:
[0021] (1) In the presence of inorganic bases and iodides, amino-protected L-tyrosine alkyl esters (SM1) react with ethyl bromide in reaction solvent I for 4-6 hours at room temperature, filter, and distill the filtrate to remove the solvent Obtain compound V;
[0022]
[0023] (2) React compound V and sodium borohydride in reaction solvent II at room temperature for 1~3h, distill off the solvent under reduced pressure, dissolve the residue in water, add ethyl acetate for extraction, separate the ethyl acetate phase, and distill out under reduced pressure Solvent obtains compound IV;
[0024]
[0025] (3) In the presence of fuacid, compound IV and alkylsulfonyl chloride (R 2 -SO 2 Cl) react in reaction solvent III at room temperature for 1.5~2.5h, filter to obtain a solution of compound III; add ethylenediamine to compound III filtrate, and ...
specific Embodiment approach
[0054] The above-mentioned content of the present invention will be further described in detail below through specific embodiments, but this should not be understood as any limitation on the protection subject matter of the present invention. All technical solutions realized based on the above content of the present invention belong to the scope of the present invention.
[0055] The present invention provides general and / or specific descriptions of the materials and test methods used in the tests. While many of the materials and methods of manipulation which are employed for the purposes of the invention are well known in the art, the invention has been described here in as much detail as possible. It is clear to those skilled in the art that in the following, if not specifically stated, the operation of the present invention is conventional or well known in the art; the test materials used can be prepared by conventional test methods by those skilled in the art through prior...
Embodiment 1
[0056] Example 1, Preparation of O-ethyl-N-tert-butoxycarbonyl-L-tyrosine methyl ester (compound V)
[0057] Dissolve 30kg (102mol) N-tert-butoxycarbonyl-L-tyrosine methyl ester in 150L N,N-dimethylformamide, add 44kg (404mol) bromoethane, 56kg (405mol) potassium carbonate and 1.35 kg (8.2mol) potassium iodide, reacted at 30°C for 5h, filtered, and evaporated the organic phase to obtain 32.7kg of compound V, yield 99.7%, HPLC purity 99.5%, MS[M+H] + :324.16, 1 H NMR (600MHz, CDCl 3 ):δ=7.02(d,J=8.4Hz,2H);6.83–6.80(m,2H);4.97(d,J=7.2Hz,1H);4.55-4.52(m,2H);4.00(dd, J=8.8,13.8Hz,2H);3.71(s,3H);3.06-2.98(m,2H);1.42(s,9H);1.40(t,J=4.8Hz;3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com