Biphenyl furocoumarin compound and preparation method and application thereof
A technology of furanocoumarins and compounds, which is applied in the field of biphenyl-type furanocoumarins and their preparation, can solve the problems of toxic and side effects of chemical medicines, and that the treatment of chemical medicines cannot meet expectations, and achieve cheap and easy-to-obtain reagents, The effect of mild reaction conditions and simple operation of the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
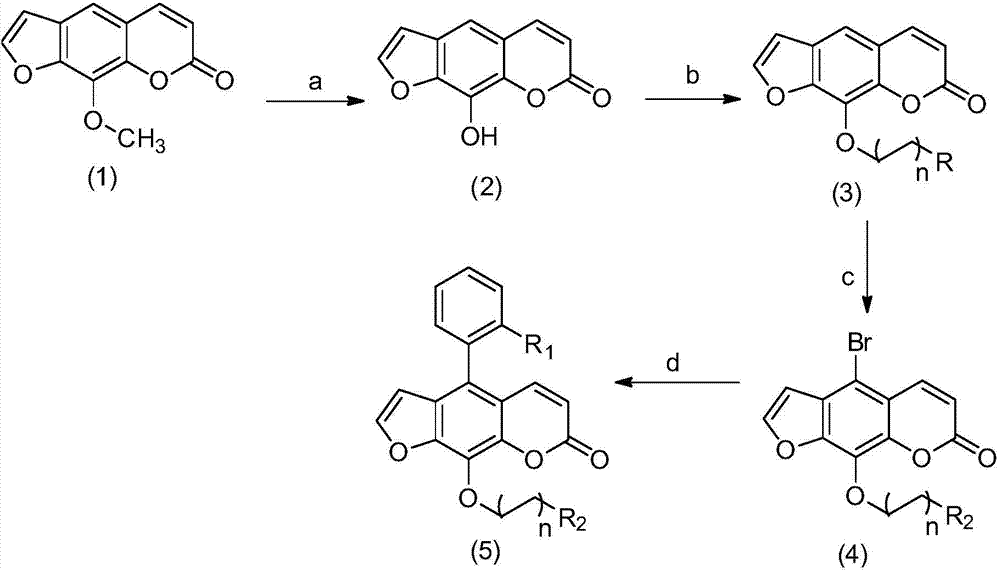
[0046] R in the structural formula of embodiment 1 1 is a fluorine atom, n is 2, R 2 Be the compound of dimethylamino, prepare by the following steps:
[0047] 1) Xanthotoxin (1) is demethylated by boron tribromide to obtain the compound Xanthoxylin (2)
[0048] Take 4.32g (20.00mmol) prickly ash toxin in a 250ml eggplant-shaped bottle, add 73ml of anhydrous dichloromethane, stir to dissolve, put it in an ice bath under nitrogen protection, and stir for 10-15min; at the same time, add 6.88ml of three Boron bromide was dissolved in 73ml of anhydrous dichloromethane to prepare a 1mol / L boron tribromide-dichloromethane solution for use.
[0049] When the temperature inside the eggplant-shaped bottle is constant, place the prepared boron tribromide-dichloromethane solution in a constant pressure dropping funnel, slowly drop it into the eggplant-shaped bottle stirred in an ice bath, and finish the dripping within 30 minutes. After the drop, the ice bath was removed, and the reac...
Embodiment 2
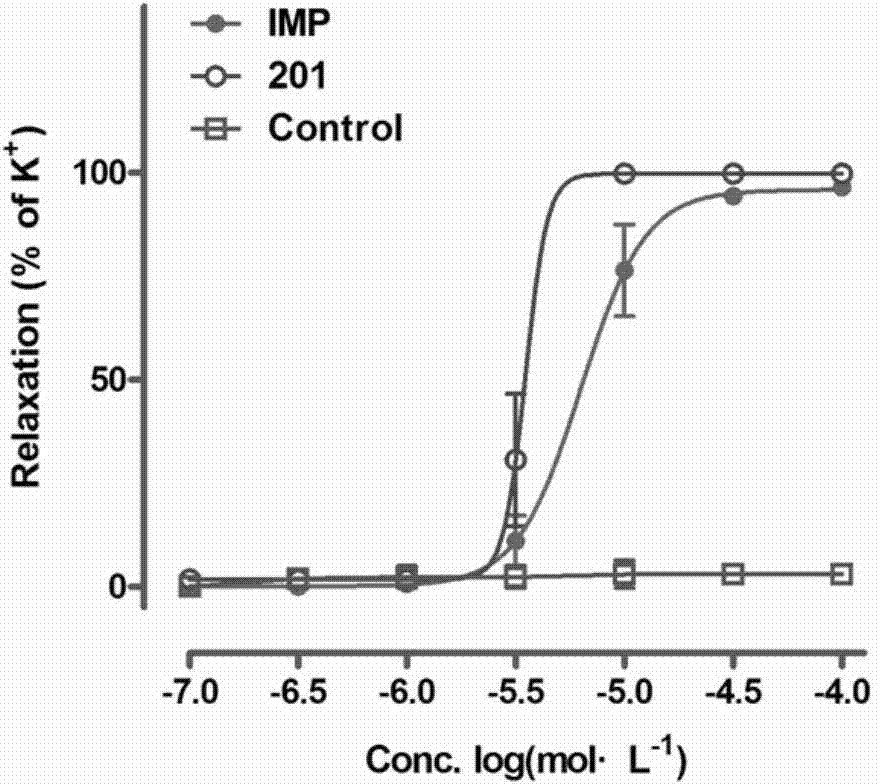
[0062] R in the structural formula of embodiment 2 1 is trifluoromethyl, n is 2, R 2 Be the compound of dimethylamino, prepare by the following steps:
[0063] Steps 1), 2), and 3) are the same preparation steps as in Example 1; Step 4) 5-bromo-9-(2-(dimethylamino)ethoxy)-7H-furan[3,2-g ] Chromene-7-one reacts with 2-trifluoromethylphenylboronic acid in a suzuki reaction, specifically:
[0064] 0.5g of 5-bromo-9-(2-(dimethylamino)ethoxy)-7H-furo[3,2-g]chromene-7-one was placed in a 50ml round bottom flask, and 20ml of 1,4-di Oxyhexane and 5ml of water were dissolved, and 0.6g of sodium carbonate, 0.25g of 2-trifluoromethylphenylboronic acid, and 0.33g of tetrakistriphenylphosphine palladium were added thereto, and the mixture was refluxed for 2.5 hours under nitrogen protection. After the reaction was completed, it was poured into water, extracted several times with a small amount of chloroform, the organic phase was dried over anhydrous sodium sulfate, the solvent was evap...
Embodiment 3
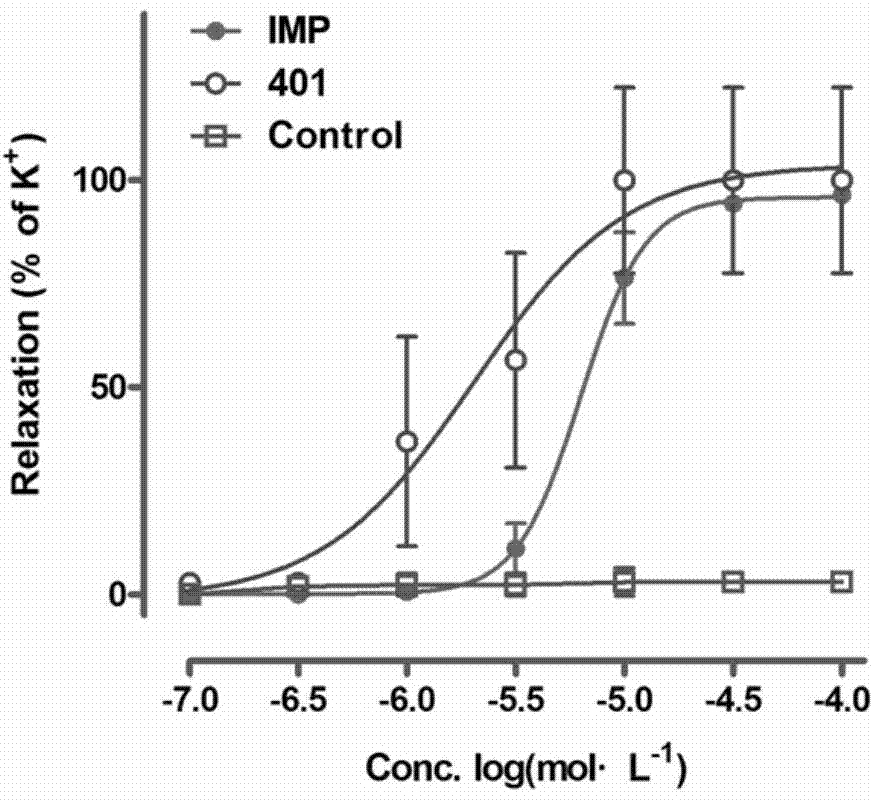
[0068] R in the structural formula of embodiment 3 1 is a fluorine atom, n is 2, R 2 Be the compound of pyrrole, prepare by the following steps:
[0069] Step 1) is the same as in Example 1, that is, the preparation steps from the compound xanthotoxin (1) to the compound xanthoxylin (2) are the same; after that, the phenolic hydroxyl group and N-(2-chloroethyl)pyrrole hydrochloride generate ether reaction, specifically:
[0070] Dissolve 0.40g (2.00mmol) of compound (2) in 10ml of treated anhydrous N,N-dimethylformamide (DMF), add 1.10g (8.00mmol) of anhydrous potassium carbonate, stir at room temperature for 30min, and then Add 0.55g (3.00mmol) of N-(2-chloroethyl)pyrrole hydrochloride, and react in an oil bath at 80°C under temperature control for 30h under nitrogen protection. After the reaction was cooled to room temperature, the system was poured into ice water and allowed to stand until the ice melted. After a small amount of extraction with chloroform several times, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com