Fluorescent nanoprobes, and preparation method and applications thereof
A fluorescent nanoprobe and nanosphere technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of cumbersome preparation of fluorescent probes, poor fluorescence stability, etc. Simple method, wide linear range effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
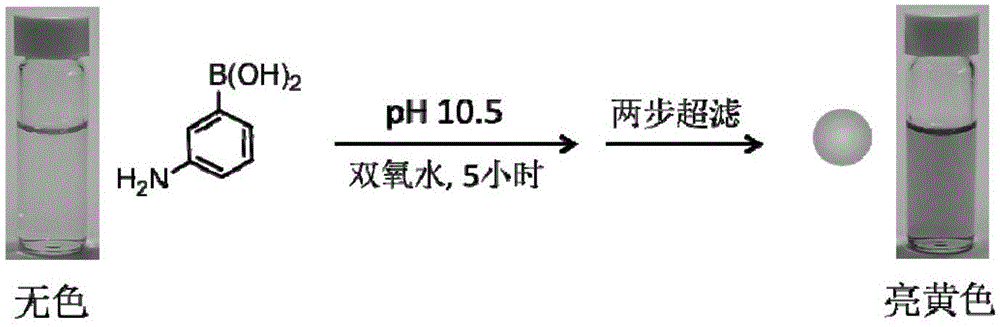
[0028] Embodiment 1: Preparation of poly-m-aminophenylboronic acid nanospheres
[0029] The reaction route is as figure 1 shown. First prepare 0.1M phosphate buffer solution (pH10.5) containing 1-10mg / mL m-aminophenylboronic acid to obtain a colorless and transparent mixed solution; then add hydrogen peroxide with a mass concentration of 30% to the mixed solution, and the addition of hydrogen peroxide The amount is 1.5 times the volume of the mixed solution, and the reaction is stirred at 25°C to 30°C for about 5 hours to obtain a bright yellow solution. The bright yellow solution is ultrafiltered with an ultrafiltration tube with a molecular weight cut-off of 50,000 Da, and the filtrate is transferred to an ultrafiltration tube with a molecular weight cut-off of 3,000 Da to continue ultrafiltration until the volume is below 200 microliters, and the obtained fraction with a molecular weight of 3,000 Da to 50,000 Da is collected. The poly-m-aminophenyl borate nanosphere powde...
Embodiment 2
[0030] Example 2: Characterization of particle size, ultraviolet absorption and fluorescence properties of poly-m-aminophenylboronic acid nanospheres
[0031] (1) Characterization of particle size distribution by dynamic light scattering
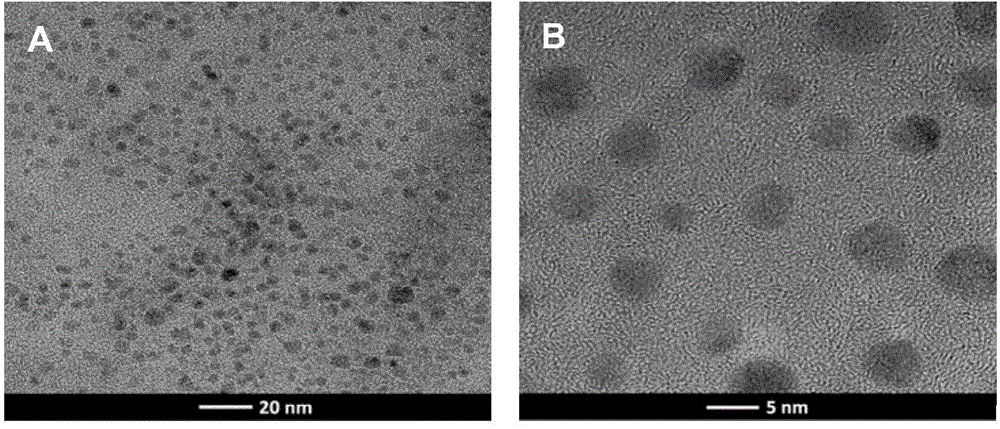
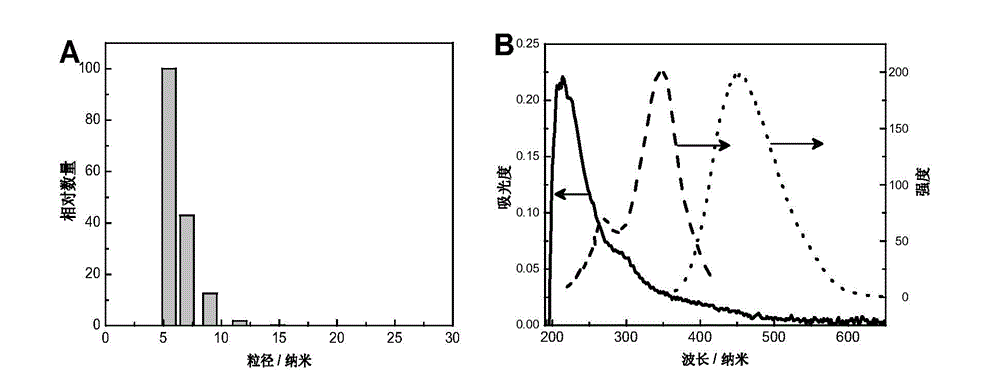
[0032] The poly-m-aminophenylboronic acid nanospheres prepared in Example 1 were dissolved in ultrapure water to prepare a solution with a concentration of 5 mg / mL, and the particle size distribution was characterized by a dynamic light scattering instrument after ultrasonication for 1 hour, and the results were as follows: image 3 a. It can be seen from the figure that the prepared poly-m-aminophenylboronic acid nanospheres have a uniform particle size distribution, and the particle size is about 5-10 nm, which is consistent with the TEM characterization.
[0033] (2) UV absorption spectrum and fluorescence spectrum
[0034] The polym-aminophenylboronic acid nanospheres prepared in Example 1 were dissolved in ultrapure water, prepared in...
Embodiment 3
[0035] Example 3: Investigation of the fluorescence stability of poly-m-aminophenylboronic acid nanospheres and fluorescent substituted boric acid
[0036] Take an appropriate amount of m-aminophenylboronic acid, p-vinylphenylboronic acid, pyridine boronic acid, pyrimidine boronic acid and poly-m-aminophenylboronic acid nanospheres respectively dissolved in 0.1M phosphate buffer (pH10.5) to make a concentration of 0.25 mg / mL solution, each solution was divided into 8 parts, and the effects of different concentrations of fructose, glucose, lysozyme, aniline, adenosine, sodium chloride and hydrogen peroxide; and pH on the fluorescence intensity of each solution were investigated. The result is as Figure 4 . It can be seen from the figure that compared with fluorescent substituted boric acid, poly-m-aminophenylboronic acid nanospheres have good fluorescence stability and are not easily affected by various conditions.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com