Recombinant ADAM15h fusion protein, preparation method and applications thereof
A fusion protein, psct-adam15h technology, applied in the field of ADAM15h and its preparation, can solve the problem that the expression of recombinant ADAM15 has not been reported, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1, the construction of the CHO cell line of recombinant ADAM15h
[0023] (1) Construction of expression vector
[0024] ADAM15 cDNA was prepared by PCR method using human spleen cDNA library, and it could be cloned into pMD18-T vector to obtain pMD-ADAM15. Nucleotide sequences were verified using sequences deposited in GenBank (Gene Bank NM_207191.1). Using this pMD-ADAM15 DNA as a template, 2130 bp ADAM15 cDNA for ADAM15 expression was prepared by PCR method with forward primer (CTGAAGCTTACCATGCGGCTGGCGCT) and reverse primer (GGATCTAGATTTAATGGTGATGATGGTGGTGATGGTGGTGATGAGCTGTGGTCAGGGAGCTGGTT). The upstream primer includes a HindIII restriction site sequence for cloning, and the downstream primer contains a 10His tag and an XbaI site sequence. The cDNA was cloned into the pSCT vector (the vector carries the dhfr and neo genes) to prepare pSCT-ADAM15h, and the sequence of ADAM15h was verified again by gene bank sequence.
[0025] (2) Transfection of pSCT-ADA...
Embodiment 2
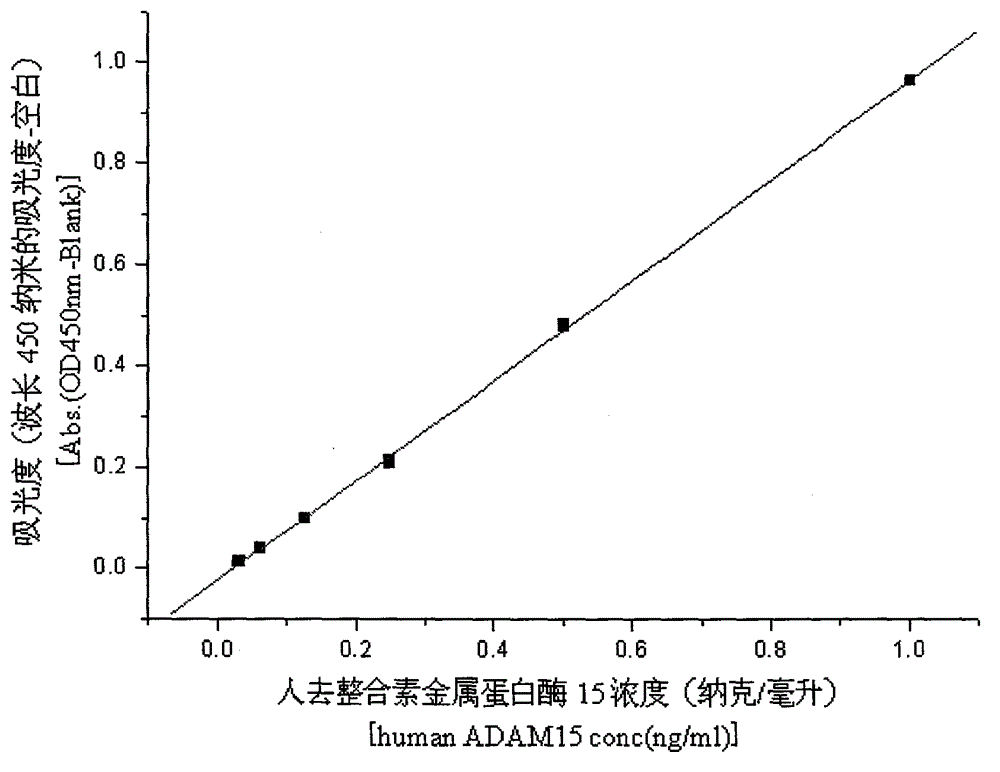
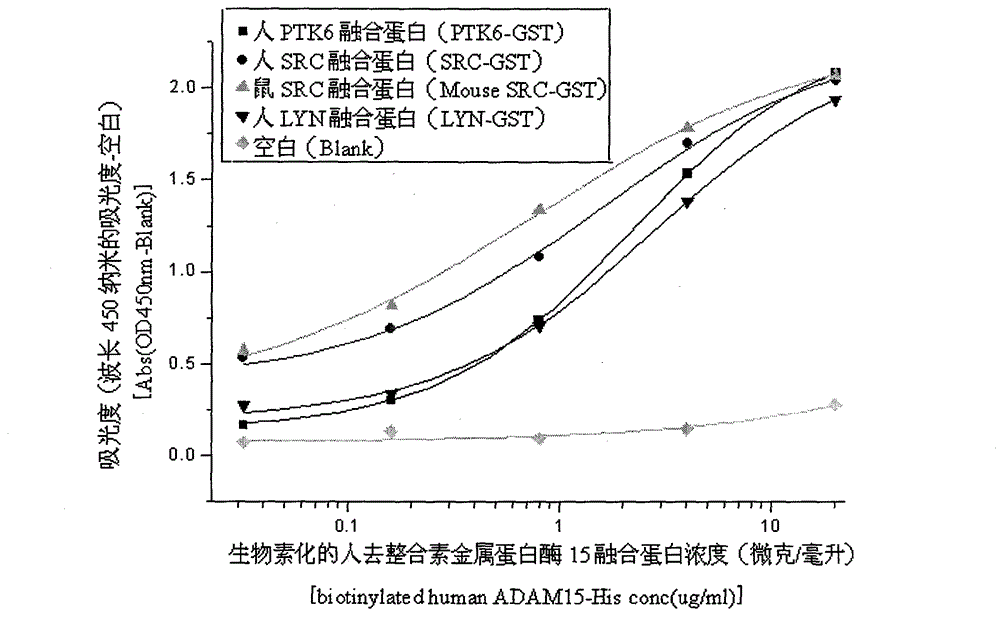
[0037] Example 2, ELISA detection of recombinant human ADAM15
[0038] The protein concentration of recombinant human ADAM15 was detected by ELISA method. The protein and antibody required for this experiment were all from Sino Biological Inc. The specific operation steps are as follows:
[0039] (1) Dilute the rabbit anti-human ADAM15 monoclonal antibody to 2ug / ml with the coating solution and coat on the microtiter plate, 100ul / well, overnight at 4°C.
[0040] (2) Discard the liquid in the well, spin dry, add washing solution at 300ul / well, and wash the plate twice.
[0041] (3) Add blocking solution at 300ul / well and react at room temperature for 1 hour.
[0042] (4) Repeat step (2).
[0043] (5) Dilute the recombinant human ADAM15 standard to 1ng / ml, 0.5ng / ml, 0.25ng / ml, 0.125ng / ml, 0.0625ng / ml and 0.03125ng / ml, and dilute the ADAM15 sample accordingly. The final samples were spotted at 100ul / well and reacted at room temperature for 2 hours.
[0044] (6) Dilute the HRP...
Embodiment 3
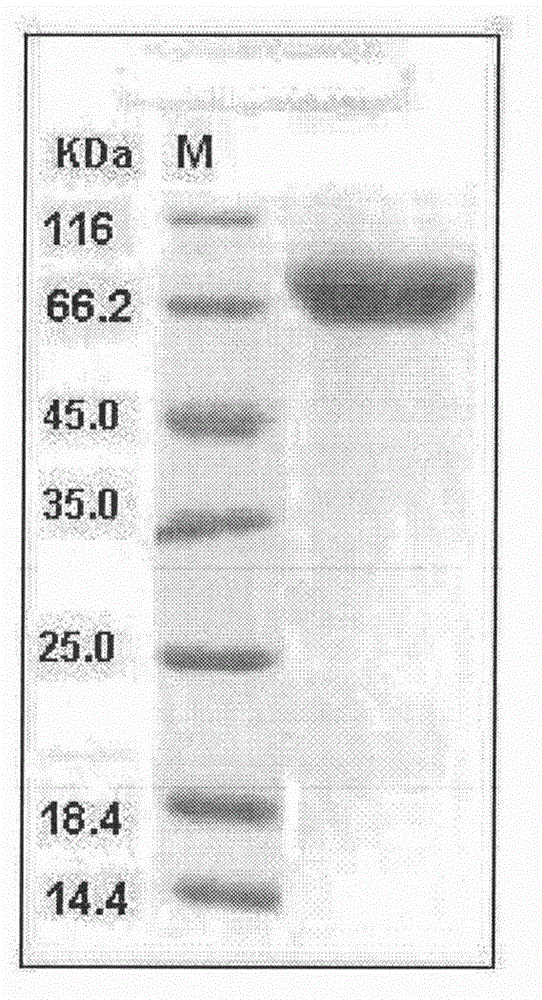
[0050] Embodiment 3, the purification of recombinant ADAM15h
[0051] Recombinant ADAM15h protein was affinity purified with Ni column, the specific method is:
[0052] (1). Balance column: 5 column volume balance buffer (PH7.0 20mM PBS 500mM Nacl 20mM imidazole 0.4mM PMSF 10% glycerol) balance Ni column.
[0053] (2). Load the sample, wash the Ni column with 5 column volume equilibration buffer.
[0054] (3). Elution: 5-10 column volumes of Elution Buffer (B1: pH7.0 20mM PBS 500mM Nacl 50mM imidazole 0.4mM PMSF 10% glycerol; B2: pH7.0 20mM PBS 500mM Nacl 500mM imidazole 0.4mM PMSF 10% glycerol) to elute the target protein.
[0055] (4). After all the samples have completed the above operations, take the samples for electrophoresis. For electrophoresis results, see figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com