Duloxetine hydrochloride controlled-release tablet and preparation method thereof
A technology for duloxetine hydrochloride and controlled-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, sugar-coated pills, etc., and can solve the problem that duloxetine hydrochloride enteric-coated preparations cannot be released at a constant rate and start to release and other problems, to achieve the effect of being suitable for industrial production, easy to operate, and simple in process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
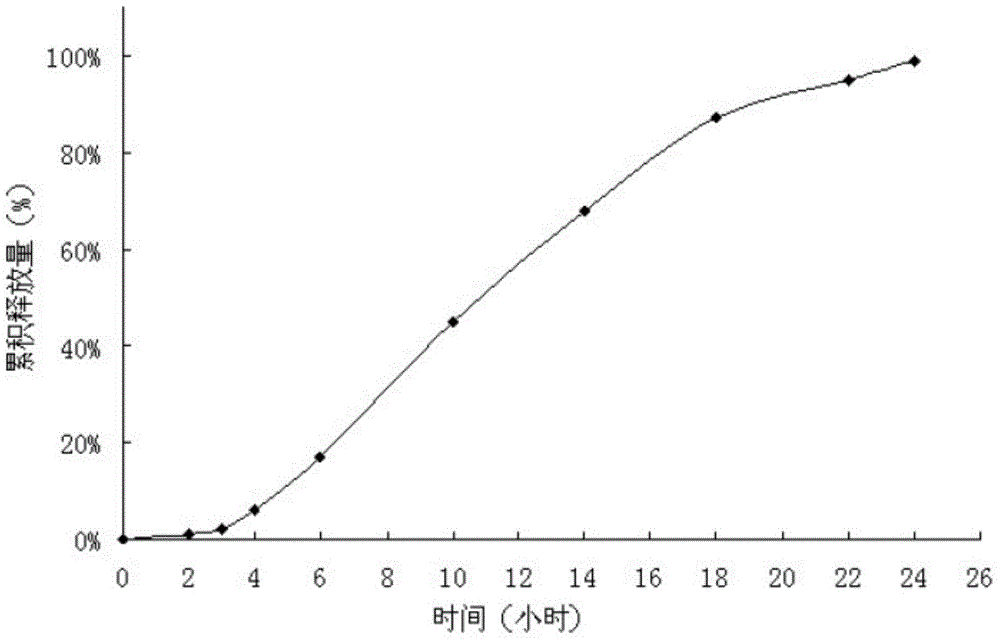
Examples
preparation example Construction
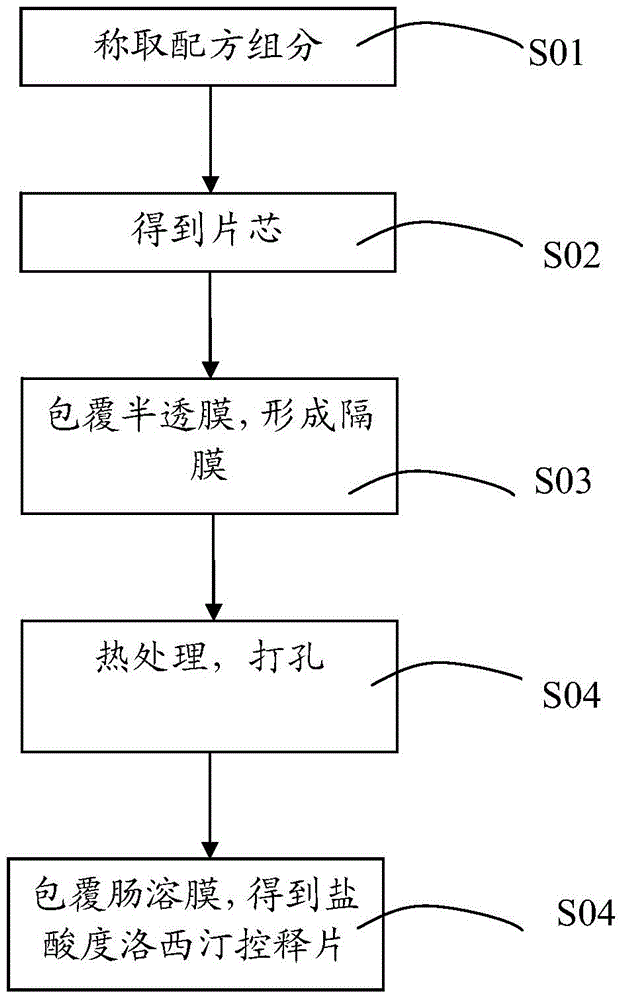
[0043] see figure 2 , figure 2 Show the schematic diagram of the preparation method of duloxetine hydrochloride controlled-release tablets in the embodiment of the present invention, including the following steps:
[0044] S01, weighing each formula component.
[0045] S02, preparing the tablet core: obtaining the above-mentioned granules of the drug-containing layer and the granules of the booster layer, and compressing the above-mentioned granules of the drug-containing layer and the granules of the booster layer to obtain a tablet core.
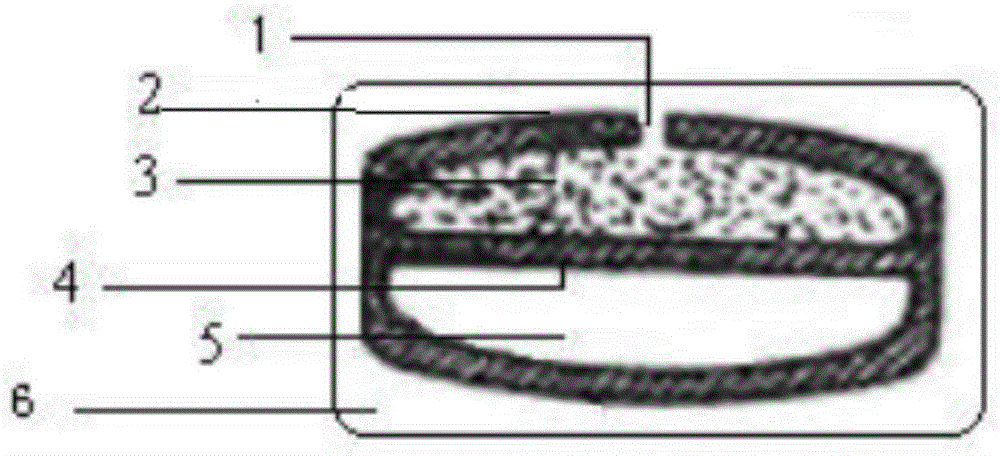
[0046] S03, coated with a semipermeable membrane: obtain the solution of the above semipermeable membrane, add the tablet core, coat the outer surface of the tablet core with a semipermeable membrane coating, and form a diaphragm between the drug-containing layer and the booster layer;
[0047] S04, punching: heat treatment, punching holes on the above-mentioned semi-permeable membrane to form through holes;
[0048] S05, coated enter...
Embodiment 1
[0070] The duloxetine hydrochloride controlled-release tablet of the present embodiment 1 has the following components in weight percentage:
[0071] Drug-containing layer composition:
[0072] Duloxetine hydrochloride 60mg, polyoxyethylene 50mg, sodium chloride 50mg, magnesium stearate 20mg, ethanol in proper amount;
[0073] Boost layer:
[0074] 250mg of polyoxyethylene, 30mg of sucrose, 20mg of magnesium stearate, appropriate amount of ethanol;
[0075] Semi-permeable membrane:
[0076] Cellulose acetate 80mg, polyethylene glycol 40mg, ethanol 5ml;
[0077] Enteric polymer film:
[0078] Eukit L30D-55 200mg, triethyl citrate 25mg, talcum powder 30mg;
[0079] The preparation method of duloxetine hydrochloride controlled release tablet of the present invention comprises the following steps:
[0080] S11, weighing each of the above formula components;
[0081] S12, preparation of tablet core:
[0082] 1. Obtain drug-containing layer particles:
[0083] (1) above-men...
Embodiment 2
[0107] The duloxetine hydrochloride controlled-release tablet of the present embodiment 2 has the following components in weight percentage:
[0108] Drug-containing layer composition:
[0109] Duloxetine hydrochloride 60mg, hypromellose 50mg, sucrose 50mg, sodium benzoate 20mg, appropriate amount of ethanol;
[0110] Boost layer:
[0111] Carbomer 250mg, mannose 30mg, talcum powder 20mg, ethanol amount;
[0112] Semi-permeable membrane:
[0113] Ethyl cellulose 80mg, hydroxypropyl cellulose 40mg, ethanol 5ml;
[0114] Enteric polymer film:
[0115] Eukit L100-55200mg, tributyl citrate 25mg, glyceryl monostearate 30mg;
[0116] The preparation method of duloxetine hydrochloride controlled release tablet of the present invention comprises the following steps:
[0117] S21, weighing each of the above formula components;
[0118] S22, preparation of tablet core:
[0119] 2. Obtain drug-containing layer particles:
[0120] (1) Sieve the above-mentioned duloxetine hydrochl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com