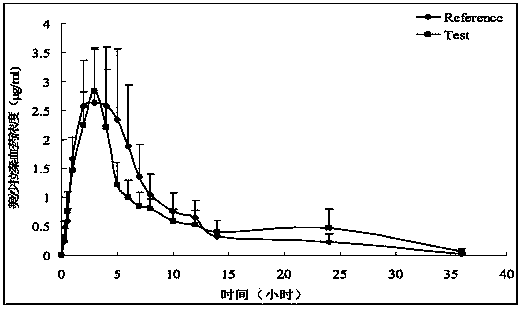
Mesalazine slow-release granules and preparation method thereof
A technology of sustained-release granules and mesalazine, which is applied in the field of new drug preparations, mesalazine sustained-release granules, and can solve problems such as limited clinical application, patients' intolerance, and small coverage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Prescription:
[0053] Mesalazine raw material 250g
[0054] Sucrose core (0.4-0.6mm) 300g
[0055] Adhesive 1
[0056] Coating Solution 1
[0057] Adhesive 2 Eudragit NE30D 76g got the second pill
[0058] Pure water 152g
[0059] Take the second upper pill 700g
[0060] Coating solution 2 Suli water (ethylcellulose microcell water dispersion) 80g
[0061] Micronized silica gel 4.8g
[0062] Pure water 400g
[0064] Preparation:
[0065] Sieving: Pass the raw materials of mesalazine through a 20-mesh sieve and set aside.
[0066] Adhesive preparation:
[0067] The first application of adhesive: Put 193.5g of pure water into a clean beaker, slowly add 6.6g of PEG-6000 (plasticizer) into the pure water evenly, stir while adding, until the solution is clear , add 38.75gS-100 (adhesive) again, cross 80 mesh sieves, both obtained the first step drug-coating adhesive.
[0068] Adhesive for the second application: put 152g of pure w...
Embodiment 2
[0077] Prescription:
[0078] Mesalazine raw material 250g
[0079] Blank starch pellets (0.4-0.6mm) 300g
[0080] Adhesive 1
[0081] Coating solution 1
[0082] Adhesive 2 Gum Arabic 73g Get second pill
[0083] Pure water 146g
[0084] Take the second upper pill 700g
[0085] Coating Solution 2 Eudragit NE30D 95g
[0087] Pure water 1900g
[0089] Preparation:
[0090] According to the preparation process described in Example 1, the packaging specification is 0.5g / bag in an aluminum-plastic composite bag.
Embodiment 3
[0092] Prescription:
[0093] Mesalazine raw material 250g
[0094] Microcrystalline cellulose pellets (0.4-0.6mm) 300g
[0095] Adhesive 1
[0096] Coating Solution 1
[0097] Adhesive 2 Polyvinylpyrrolidone (PVP) 54.8g Get the second pill
[0098] Pure water 189g
[0099] Take the second upper pill 700g
[0100] Coating Solution 2 Eudragit NE30D 87.4g
[0101] Micronized silica gel 4.5g
[0102] Pure water 1748g
[0103] Glidant talcum powder 80g
[0104] Preparation:
[0105] According to the preparation process described in Example 1, the packaging specification is 0.5g / bag in an aluminum-plastic composite bag.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com