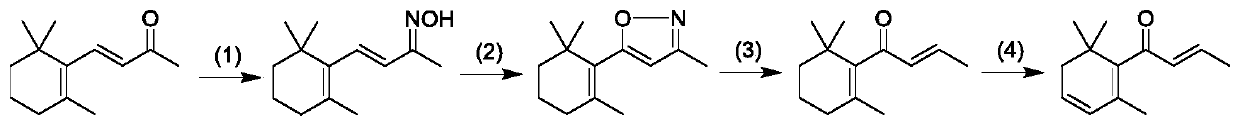
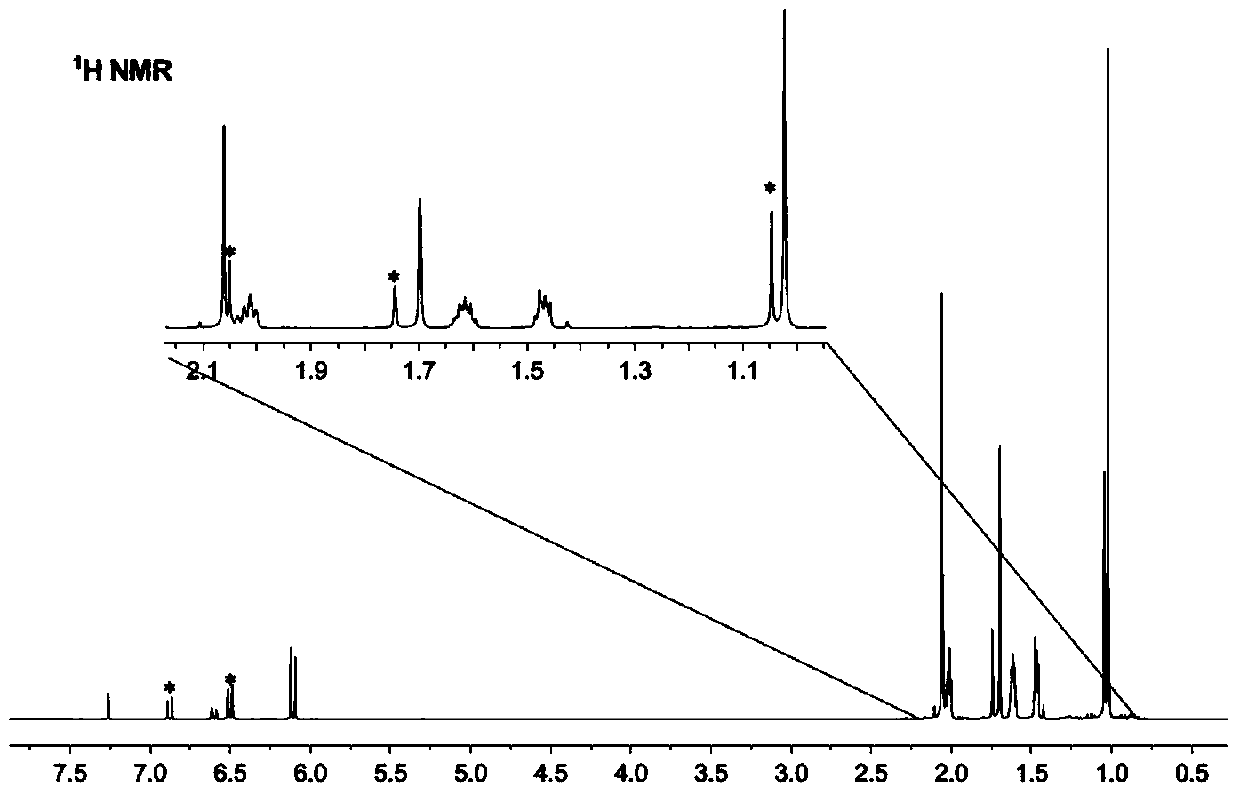
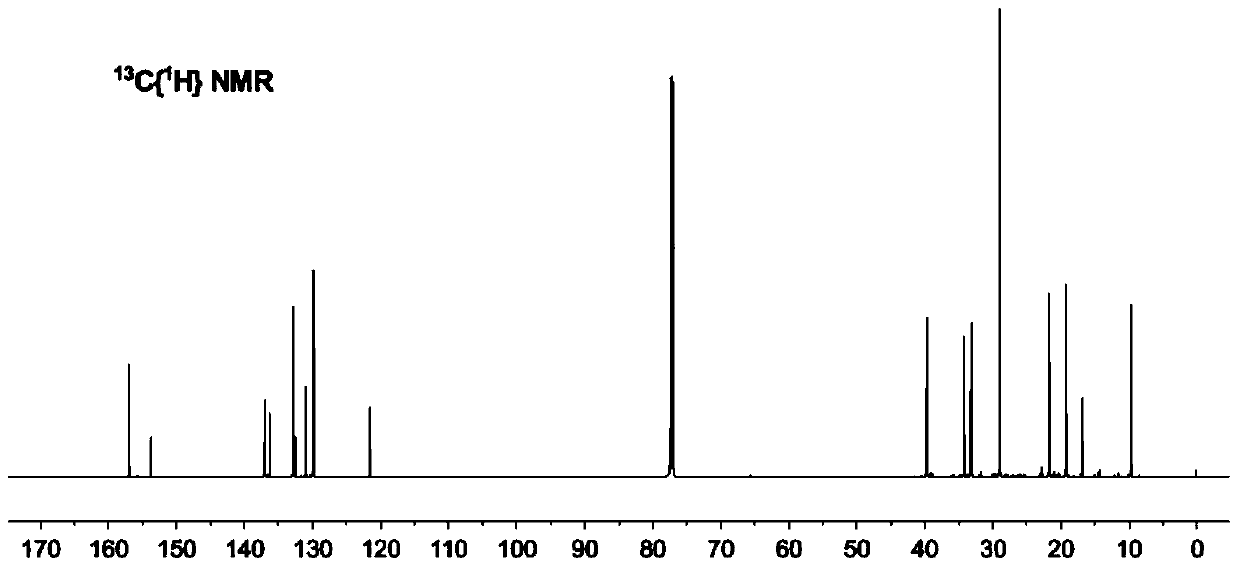
Preparation method of beta-damascenone spice
A damascenone fragrance and raw material technology, applied in the field of preparation of β-damascenone fragrance, can solve the problems of unsuitability for industrial production, harsh reaction conditions, high cost, etc., and achieve low cost, short synthesis route and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Synthesis of β-ionone oxime
[0044] Add 3.85g (0.06mol) of hydroxylamine hydrochloride, 6.25g (0.07mol) of sodium acetate, and 10mL of distilled water into a 50mL round-bottomed flask, stir and add 10.00g (0.05mol) of β-ionone and 20mL of ethanol. Heating in a water bath, stirring the reaction for 2h and ending the reaction. Extract with diethyl ether, extract three times, combine the extracts, wash with 10mL of 10% sodium bicarbonate, and then dry, suction filter and rotary evaporate to finally obtain β-ionone oxime with a yield of about 99.0%.
Embodiment 2
[0045] Example 2: Synthesis of β-violet isoxazole derivatives
[0046] Add 26.50g (0.19mol) of potassium carbonate, 100mL of distilled water, 50mL of tetrahydrofuran and 10.00g (0.05mol) of β-ionone oxime into a 250mL round bottom flask, and add 12.20g (0.05mol) of iodine and 27.60 g (0.17 mol) potassium iodide. Reflux at 66°C for 6h, extract with ether, extract 3 times, wash with 7.5mL saturated sodium sulfite solution at the same time, combine the extracts, then dry, filter and rotary evaporate to obtain β-violet isoxazole derivatives, the yield About 89.5%.
Embodiment 3
[0047] Embodiment three: the synthesis of β-dihydrodamascenone
[0048] Vacuumize the 500mL three-neck round bottom flask first, then keep it at a low temperature of -40°C, and pass ammonia gas into it until there is about 100mL of liquid ammonia in the round bottom flask, then stop the flow of ammonia gas. At room temperature, add 5.00g (0.024mol) of β-violet isoxazole derivatives, 58mL of tetrahydrofuran and 5.38mL (0.07mol) of tert-butanol into about 100mL of liquid ammonia, and add metal sodium fragments under stirring Until the solution turns blue, the stirring reaction is continued for 15 min after turning blue. Add NH to the reaction solution 4 Cl until the solution turns white. Extracted with ether, anhydrous Na 2 SO 4 Dry for 12h. Then through suction filtration, rotary steaming. 40 mL of toluene was added to the concentrated solution, and the mixed solution was refluxed and stirred at 120° C. for 24 hours, and then rotary-evaporated to obtain the final product ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com