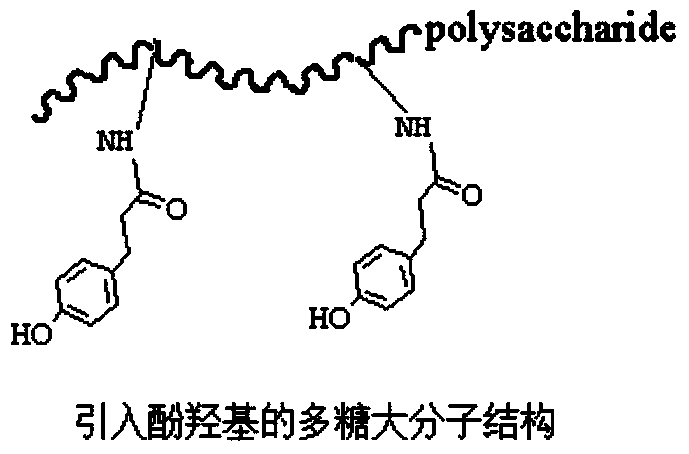
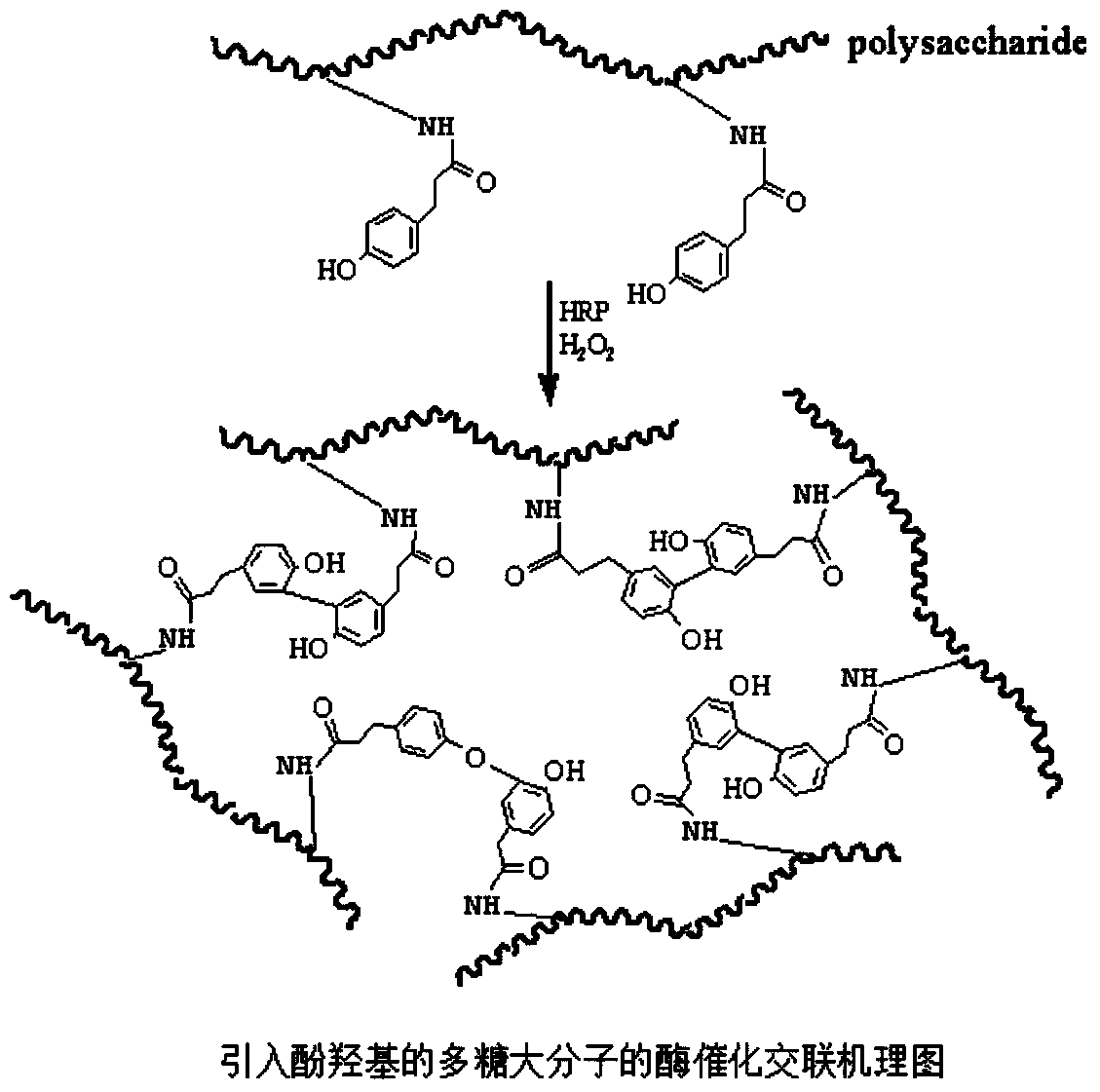
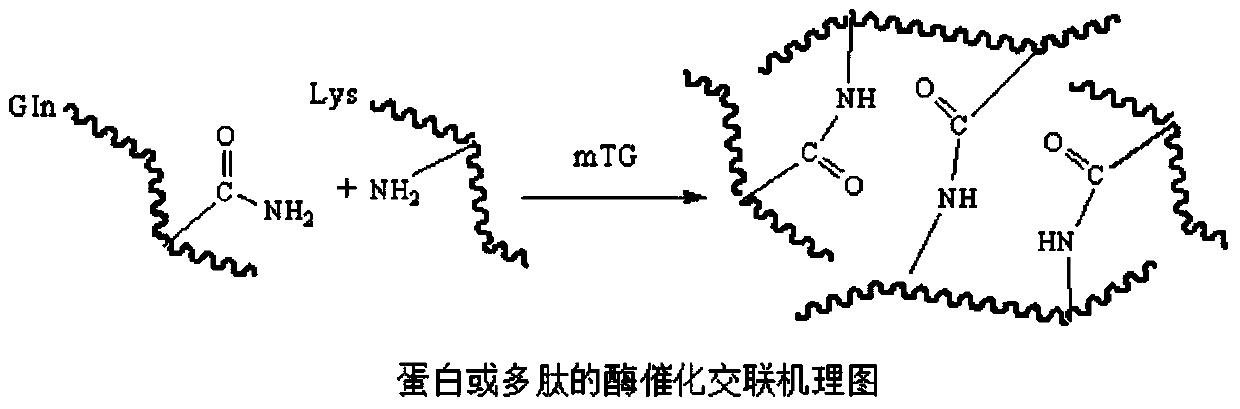
Biomacromolecule interpenetrating polymer network hydrogel and preparation method thereof
A biomacromolecule and interpenetrating network technology, applied in the field of biomedical polymer materials, can solve the problems of reduced biocompatibility, poor mechanical strength, poor physical cross-linking effect, etc., to overcome toxicity and achieve excellent biocompatibility. Sex, resolving ineffective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Preparation of chitosan-p-hydroxyphenylpropionic acid (CHPA)
[0049] Water-soluble chitosan (0.88g, 5mmol) was weighed and dissolved in 150ml of deionized water, and stirred at constant temperature with a magnetic stirrer until completely dissolved. Weigh p-hydroxyphenylpropionic acid (1.66g, 10mmol), 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl) (2.73g, 15mmol), N -Hydroxysuccinimide (NHS) (1.72g, 15mmol) was dissolved in 100ml of N,N-dimethylformamide (DMF) aqueous solution, wherein DMF:water (3:2, V:V), activated at room temperature 1h (pH=4.7), the activation solution was added to the chitosan solution, and stirred overnight at room temperature. The reaction solution was moved into the dialysis bag for dialysis with distilled water for 3 days, and the dialysate was adjusted to pH to separate out the product, and vacuum drying was performed to obtain the chitosan of the product bonded p-hydroxyphenylpropionic acid, hereinafter referred to as ...
Embodiment 2
[0051] Preparation of hyaluronic acid-tyramine (HA-Tyr)
[0052] Weigh hyaluronic acid (2g, 5mmol) and dissolve it in 100ml of deionized water, stir with a magnetic stirrer at constant temperature until completely dissolved. Weigh tyramine (1.68g, 10mmol), EDC·HCl (2.73g, 15mmol), NHS (1.72g, 15mmol) dissolved in 100ml of distilled water, activate at room temperature for 1h (pH=4.7), add the activation solution to the transparent acid solution, stirred overnight at room temperature. The reaction solution was transferred into a dialysis bag and dialyzed with distilled water for 3 days, the pH of the dialysate was adjusted to precipitate the product, and the product was vacuum-dried to obtain hyaluronic acid bound to tyramine, hereinafter referred to as hyaluronic acid-tyramine.
Embodiment 3
[0054] Preparation of Heparin-Tyramine (Hep-Tyr)
[0055] Weigh heparin (0.5 g) and dissolve it in 100 ml of deionized water, stir with a magnetic stirrer at constant temperature until completely dissolved. Weigh tyramine (1.68g, 10mmol), EDC·HCl (2.73g, 15mmol), NHS (1.72g, 15mmol) dissolved in 100ml of distilled water, activate at room temperature for 1h (pH=4.7), add the activation solution to the heparin The solution was stirred overnight at room temperature. The reaction solution was transferred into a dialysis bag and dialyzed with distilled water for 3 days, the pH of the dialysate was adjusted to precipitate the product, and the product was vacuum-dried to obtain heparin bound to tyramine, hereinafter referred to as heparin-tyramine.
PUM
| Property | Measurement | Unit |
|---|---|---|
| compressive strength | aaaaa | aaaaa |
| compressive strength | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com