Application of mangiferin aglycone and its derivatives in the preparation of antiprostatic hyperplasia drugs
A prostatic hyperplasia and drug technology, applied in the field of medicine, can solve problems such as quality control difficulties, unknown material basis and active ingredients of Chinese patent medicine preparations, adverse reactions of chemical drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Inhibition of 5α-reductase in vitro
[0033] 1. Experimental materials
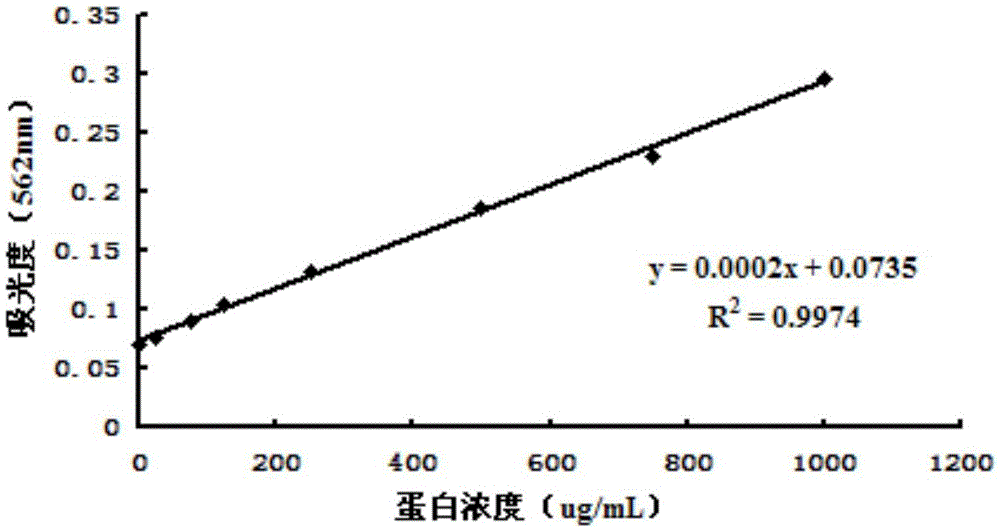
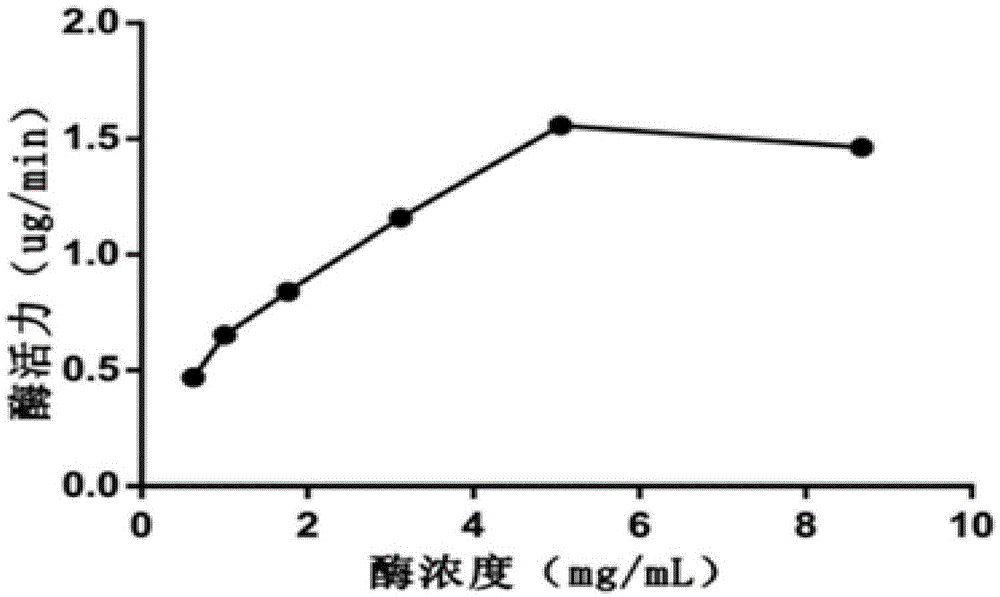
[0034] SD female mice, Kunming Medical University; Testosterone, Shanghai Yuanye Biotechnology Co., Ltd. (20130409); NADPH, sigma company (Lot SLBC6718V); Finasteride, Hubei Subang Pharmaceutical Co., Ltd. (20121001); Tris-base, BIOSHARP Company (Amresco0497); Sodium Chloride, Sangon Biological Company (Lot3004B220); DTT, Sigma Company (LotBCBK8875V); Absolute Ethanol, Yunnan Shandian Pharmaceutical Co., Ltd. (Lot LN70N21); Dichloromethane, Tianjin Windboat Chemical Reagent Technology Co., Ltd. (20100926); Chromatographic methanol, Beijing Bailingwei Technology Co., Ltd. (116481); Mangiferin; 1-hydroxy-3,6,7-triacetoxymangiferin; 3-hydroxy-1,6 , 7-triacetoxymangiferin; 1,3-dihydroxy-6,7-diacetoxymangiferin; 6,7-dihydroxy-1,3-diacetoxymangiferin, purity All are greater than 98%, provided by Kunming Pharmaceutical Group Drug Research Institute.
[0035] 2. Experimental equipment
[003...
Embodiment 2
[0057] Example 2: Investigation of the In Vivo Pharmacological Effects of Testosterone Propionate Replication Prostatic Hyperplasia Model Mice
[0058] 1. Test material
[0059] 1.1 Animals
[0060] SPF-grade male ICR mice, weighing 20-22 g, were provided by the Animal Department of Kunming Pharmaceutical Group Co., Ltd., license number SCXK (Dian).
[0061] 1.2 Test substance
[0062] Mangiferin, light yellow powder, purity >98%, provided by Kunming Pharmaceutical Group Research Institute, batch number 20130903; Finasteride Tablets (Guoyao Zhunzi H20070146), 5mg / tablet, Hubei Subang Pharmaceutical Co., Ltd., batch number: 0121001 ; Longbishu Capsules (Z10960007), 0.3g / capsule, Shijiazhuang Kedi Pharmaceutical Co., Ltd., batch number: 111113; use pure water to make suspensions of different concentrations for later use.
[0063] 1.3 Reagents
[0064] Testosterone Propionate Injection (Guoyao Zhunzi H12020531), 10mg / mL, Tianjin Jinyao Amino Acid Co., Ltd., batch number 12020...
Embodiment 3
[0099] Example 3: Acute Toxicity Test by Oral Gavage in Mice
[0100] Preliminary test shows that LD cannot be detected by oral administration of mangiferin in mice 50 , so the acute toxicity of the test substance is reflected by the maximum dose administered twice a day. During the test, after the animals were fasted and given water for 8 hours, 40 animals weighing 19.5 to 21.9 g, half male and half male, were randomly divided into a treatment group and a control group according to sex and body weight, with 10 male and 10 males in each group. The mice in the treatment group were given 0.27g / mL mangiferin paste by oral gavage at the maximum capacity of 40mL / kg, and then administered again after an interval of 6h. The equivalent dose was 21.6g / kg.d, and the control group was given the same volume of pure water. As a result, there were no obvious toxic reactions in the appearance, behavior, mental state, appetite, urine and stool, fur, skin color and breathing of the two group...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com