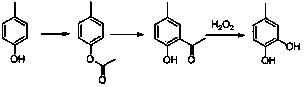
Method for preparing 4-methyl catechol
A technology of methyl catechol and p-cresol, which is applied in the preparation of 4-methyl catechol and the field of 4-methyl catechol, can solve the problems of low yield and pollution of the preparation method, Achieve the effects of high yield, easy access to raw materials, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment l
[0026] 1) The 500ml three-necked flask is equipped with a stirrer, a thermometer, and a detachable reflux condensing device. Add 108g (1000mmol) p-cresol, then add 109g (1068mmol) acetic anhydride, heat with an adjustable pressure electric heating mantle, reflux reaction under stirring for 6.0 hours, change the reflux device to a vacuum distillation device, and steam the acetic acid and Excessive acetic anhydride, phenolic ester yield 98%.
[0027] p-cresol acetate, 1 HNMR (500MHz, CDCl3): δ(ppm)=7.21 (d, J = 8.2 Hz, 2H), 7.01 (d, J = 8.3 Hz, 2H), 2.38 (s, 3H), 2.32 (s, 3H).
[0028] 2) In a 500ml four-necked flask equipped with a stirrer, a thermometer, a detachable reflux condensing device, a constant pressure dropping funnel, passing through N2, tail gas absorption and other devices, add 120g (1066mmol) of pre-dewatered chlorobenzene, and stir Add 138g (1035mmol) of anhydrous aluminum trichloride, heat to 130~140°C, and drop in the product p-cresol acetate in 1). Continu...
Embodiment 2
[0033] 1) As described in Example 1, the ratio of p-cresol and acetic anhydride added is 1.0:3.0. The reaction was refluxed for 2.5 hours under stirring, and the yield was 99%.
[0034] 2) As described in Example 1, the inert solvent used was 150g of xylene, heated to 135~145°C, phenolic ester was added dropwise, and the reaction was stirred for 2.5h, wherein the molar ratio of phenolic ester to aluminum tribromide was 1.0:2.0, The molar ratio of phenolic ester to dilute acid is 1.0:2.5, the post-treatment temperature is 40℃, and the yield is 92%.
[0035]3) As described in Example 1, 2-hydroxy-5-methylacetophenone was dissolved in 15 times the mass of ethanol, and slowly dropped into a 20% potassium hydroxide solution at 15-20°C, wherein The molar ratio of sodium hydroxide to phenolic ketone is 1.5. Regulate the system by adding 15% hydrogen peroxide dropwise at -10 to -5°C, wherein the molar ratio of hydrogen peroxide to phenolic ketone is 2.0:1.0. Drop lye to maintain an...
Embodiment 3
[0037] 1) As described in Example 1, the ratio of p-cresol and acetyl chloride added is 1.00:1.01. The reaction was refluxed for 4.5 hours under stirring, and the yield was 92%.
[0038] 2) As described in Example 1, the inert solvent used is toluene 275g, heated to 110~120°C, phenol ester is added dropwise, stirred and reacted for 15 hours, wherein the molar ratio of phenol ester to aluminum chloride is 1:3, phenol ester The molar ratio with dilute acid is 1:5, the aftertreatment temperature is 20°C, and the yield is 86%.
[0039] 3) As described in Example 1, dissolve 2-hydroxy-5-methylacetophenone in 3 times the mass of dioxane, and slowly drop into a sodium carbonate solution with a mass fraction of 40% at 25~30°C , wherein the molar ratio of sodium hydroxide to phenolic ketone is 2.5. Regulate the system by adding 5% hydrogen peroxide dropwise at 0-5°C, wherein the molar ratio of hydrogen peroxide to phenolic ketone is 2.8:1.0. Drop lye to maintain an alkaline pH value...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com