Method for preparing methyl cedryl ketone from Chinese fir wood oil
A technology of methyl cedryl ketone and fir oil, which is applied to the preparation of carboxylic acid esters, carbon-based compounds, chemical instruments and methods, etc., can solve the problems of scarcity of cedar resources, long growth cycle of cedar wood, and difficulty in cedar wood oil, etc., to achieve Avoid the hazards of production operators and the environment, facilitate the range of fragrance blending, and facilitate separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] The preparation of niobic acid supported aluminum chloride catalyst in each embodiment:
[0033] Soak 250 grams of niobic acid in 1 mol / L nitric acid solution at room temperature, take it out after 24 hours, then dry it at 120°C for 5 hours, and then bake it at 350°C for 3 hours to obtain pore-expanding niobic acid.
[0034] Add 250 g of pore-expanding niobic acid to 1 liter of saturated solution of aluminum chloride at 70°C in absolute ethanol, heat and reflux for 2.5 h, filter with suction, wash with ethanol until no chloride ions are detected, and dry at 105°C for 2 h , ground through a 120-mesh sieve, solidified in a muffle furnace at 350° C. for 2 hours, cooled at room temperature and placed in a desiccator to obtain a niobic acid-supported aluminum chloride catalyst. The used niobic acid-supported aluminum chloride catalyst was regenerated by calcining at 300° C. for 2 h in a muffle furnace, and the regenerated catalyst was used in the synthesis reaction of methyl...
Embodiment 1
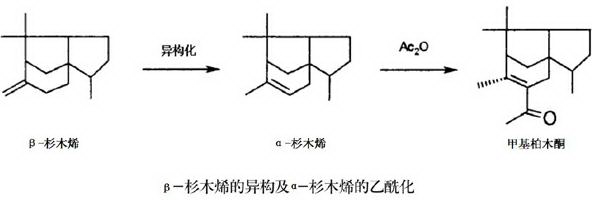
[0036] Chinese fir oil is distilled to obtain firne, which mainly contains α-pinene, β-pinene and α-terpineol.
[0037] Add 210 grams of fircene and 20 grams of niobic acid-loaded aluminum chloride catalyst into the reaction kettle, stir and heat up to 55°C for isomerization reaction for 2 hours, β-pinene is converted into α-pinene, and then start to drop 410 grams of acetic anhydride , After 30 minutes of dropwise addition, stir and heat up to 60°C for acetylation reaction for 5 hours, α-pinene is converted into methyl cedryl ketone through acetylation, and α-terpineol is converted into terpineyl acetate through acetylation. After the reaction, the product was rectified first to distill out acetic anhydride and acetic acid, and then an appropriate amount of crushed ice was added to keep the reaction mixture below 40°C. Stirring was continued for 0.5 h, and the layers were allowed to stand to separate the oil layer and the water layer. % sodium carbonate solution until weakly al...
Embodiment 2
[0039] Add 210 grams of picrene and 15 grams of niobic acid-supported aluminum chloride catalyst into the reaction kettle, stir and heat up to 55°C for isomerization reaction for 2 hours, β-pinene is converted into α-pinene, and then start to drop 320 grams of acetic anhydride , After 30 minutes of dropwise addition, the temperature was raised to 60°C for 7 hours for acetylation reaction with stirring, α-pinene was acetylated into methyl cedryl ketone, and α-terpineol was acetylated into terpineyl acetate. After the reaction, the product was rectified first to distill out acetic anhydride and acetic acid, and then an appropriate amount of crushed ice was added to keep the reaction mixture below 40°C. Stirring was continued for 0.5 h, and the layers were allowed to stand to separate the oil layer and the water layer. % sodium carbonate solution until weakly alkaline, then washed with saturated salt and deionized water until neutral, and the water layer was separated. The washed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com