Preparation method of L-serine
A technology of serine and nitric acid, which is applied to the preparation of organic compounds, cyanide reaction preparation, chemical instruments and methods, etc., can solve the problems of difficult industrial production, troublesome operation, expensive enzymes, etc., so that the reaction conditions are not harsh and reduce The effect of cost and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
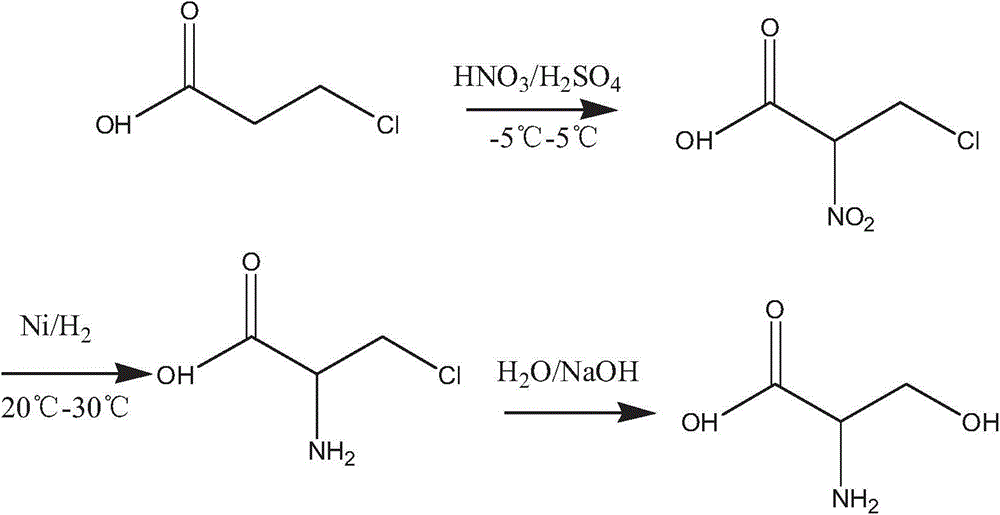
[0017] Preparation of 2-nitro-3-chloropropionic acid
[0018] Concentrated H 2 SO 4 160-200ml in a 500ml three-necked bottle equipped with a thermometer, stir and cool down to below 0°C to control the temperature, 20ml of β-chloropropionic acid, add 60-100ml of water dropwise, and then add 40% concentrated HNO 3 10.5ml~11.5ml, react at -2~1℃ for 2~3h. After the reaction was completed, 250ml of ice-water mixture was added, and then with CH 2 Cl 2 Extract, concentrate and crystallize, and dry to obtain about 31.5 g of the product.
Embodiment 2
[0020] Preparation of 2-amino-3-chloropropionic acid
[0021] Put 30.8g of 2-nitro-3-chloropropionic acid into a 500ml airtight three-neck bottle equipped with a thermometer, dissolve it in 100-160ml of water, add 2.5g of Ni, pass a hydrogen balloon into the solution, and keep it at room temperature at 23-28°C React overnight for 12h. After the reaction was completed, filter and concentrate the crystals. After drying, about 23g of the product was obtained.
Embodiment 3
[0023] Preparation of L-serine
[0024] Prepare NaOH concentration of 2mol / L, add 20g of 2-amino-3-chloropropionic acid, put it into a 250ml three-neck bottle equipped with a thermometer, stir until dissolved, heat up to 50-55°C, stir for about 4-5 hours, and react At the end, use dilute HCl to adjust the pH value to 7.25-7.35, use electrodialysis to remove salt, and finally concentrate the water phase, crystallize, filter, dry the solid, and weigh to obtain about 16 g of the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com