Ratiometric fluorescent probe for detecting hydrazine, and preparation method thereof
A fluorescent probe and ratiometric technology, applied in the field of fluorescent probes, can solve problems such as interference signal output and rare probes, and achieve the effects of good selectivity, strong anti-interference ability, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Take 0.27g (1mmol) of 4-hydroxy-N-butyl-1,8-naphthalene diimide, add it to excess acetic anhydride, heat to reflux for 8h, and cool to room temperature. The reaction solution was poured into 100 mL of ice water, a large amount of precipitates were precipitated, filtered with suction, washed with a small amount of cold ethanol, and dried in vacuum to obtain a hydrazine molecular fluorescent probe.
[0029] 1 H NMR (600 MHz, DMSO- d 6 ): δ 8.52 (q, 2H), 8.43 (q, 1H), 7.90 (q, 1H), 7.69 (d, 1H), 4.04 (t, 2H), 2.52 (s, 3H), 1.62 (m, 2H ), 1.36 (m, 2H), 0.93 (t, 3H).
[0030] 13 C NMR (150 MHz, DMSO- d 6 ): δ 172.3, 166.6, 166.1, 154.7, 134.7, 134.5, 131.9, 131.4, 131.0, 128.2, 125.7, 123.4, 123.3, 33.0, 24.2, 23.1, 17.0.
[0031] Calculated HRMS (ESI): C 18 h 17 NO 4 [1+Na] + 334.1050; Found: 334.1054.
Embodiment 2
[0033] The hydrazine molecular fluorescent probe prepared in Example 1 was dissolved in DMSO to prepare a hydrazine molecular fluorescent probe standard solution with a concentration of 10 mmol / L.
[0034] Hydrazine was prepared into a stock solution of hydrazine molecules with a concentration of 1 mmol / L with distilled water.
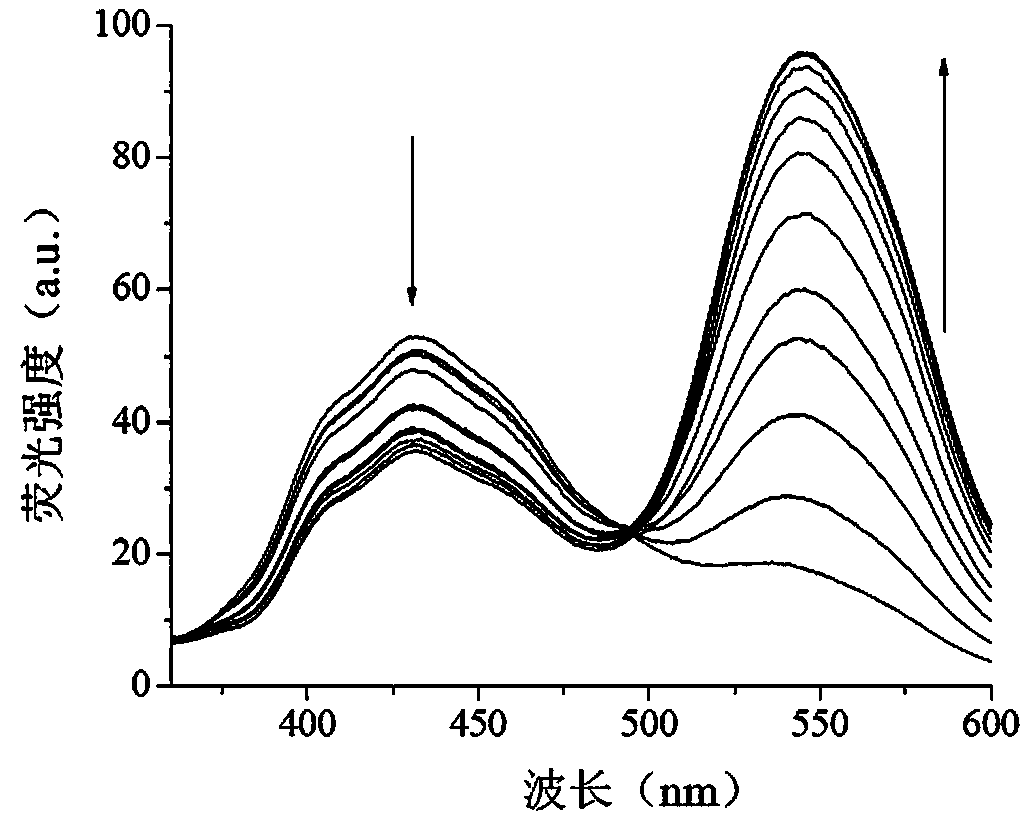
[0035] Take 4, 8, 12, 20, 24, 28, 32, 36, 40, 60, and 80 μL of hydrazine molecule stock solution, respectively, add 2 μL of hydrazine molecule fluorescent probe standard solution, and use DMSO:PBS buffer (10 mM, pH = 7.4) = 1:1 (v / v) mixed solution was diluted to 2mL, and the concentration of fluorescent probe was prepared as 1×10 -5 M, hydrazine concentration 0.2~4×10 -5 A series of detection solutions of M, let stand at room temperature for 5 minutes, measure the fluorescence emission spectra of the series of detection solutions under the condition of fluorescence excitation wavelength of 300nm and slit width of 5nm / 5nm, take the wavelength as the a...
Embodiment 3
[0038] Take the hydrazine molecule stock solution of Example 2, and dilute it with a mixture of DMSO:PBS buffer (10mM, pH=7.4)=1:1 (v / v) to prepare 2×10 -6 M, 4×10 -6 M, 6×10 -6 M, 8×10 -6 M, 1×10 -5 Hydrazine standard solution of M.
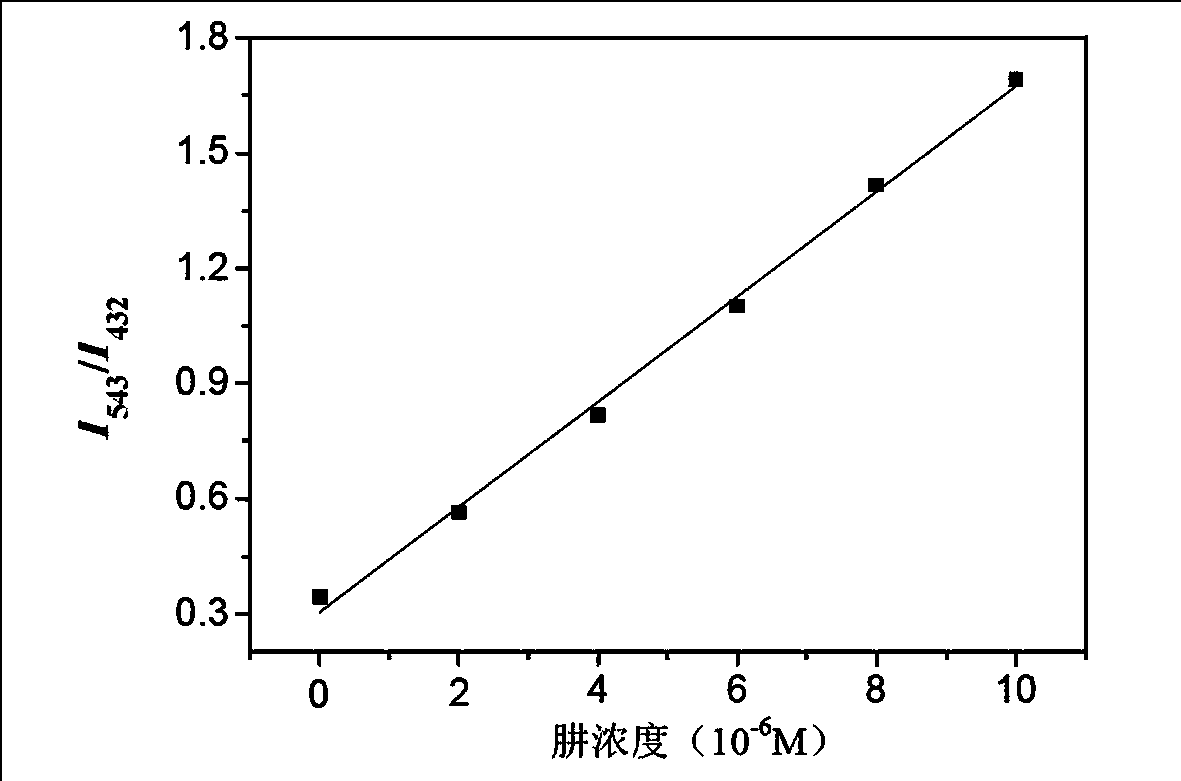
[0039] Take 2 μL of the fluorescent probe standard solution of Example 2 and add it into a fluorescent cup, dilute it to 2 mL with the above-mentioned hydrazine standard solution of different concentrations, and let stand at room temperature for 5 minutes. Detect its fluorescence intensity under 543nm and 432nm wavelength respectively, take the concentration of hydrazine standard solution as abscissa, the ratio of fluorescence intensity at 543nm and 432nm place (I 543 / I 432 ) as the ordinate, we get figure 2 The standard curve diagram of the fluorescence response of the hydrazine molecular fluorescent probe reacted with different concentrations of hydrazine is shown.
[0040] Depend on figure 2 Visible, in 0~1×10 -5 Within the concen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com