Combined desulfuration dephosphorization method
A technology for dephosphorization and hydrogen sulfide, applied in separation methods, chemical instruments and methods, and separation of dispersed particles, can solve the problems of high oxidant consumption, poor operability, corrosion of pipelines, etc., and achieve no secondary pollution, low cost, The effect of high removal efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
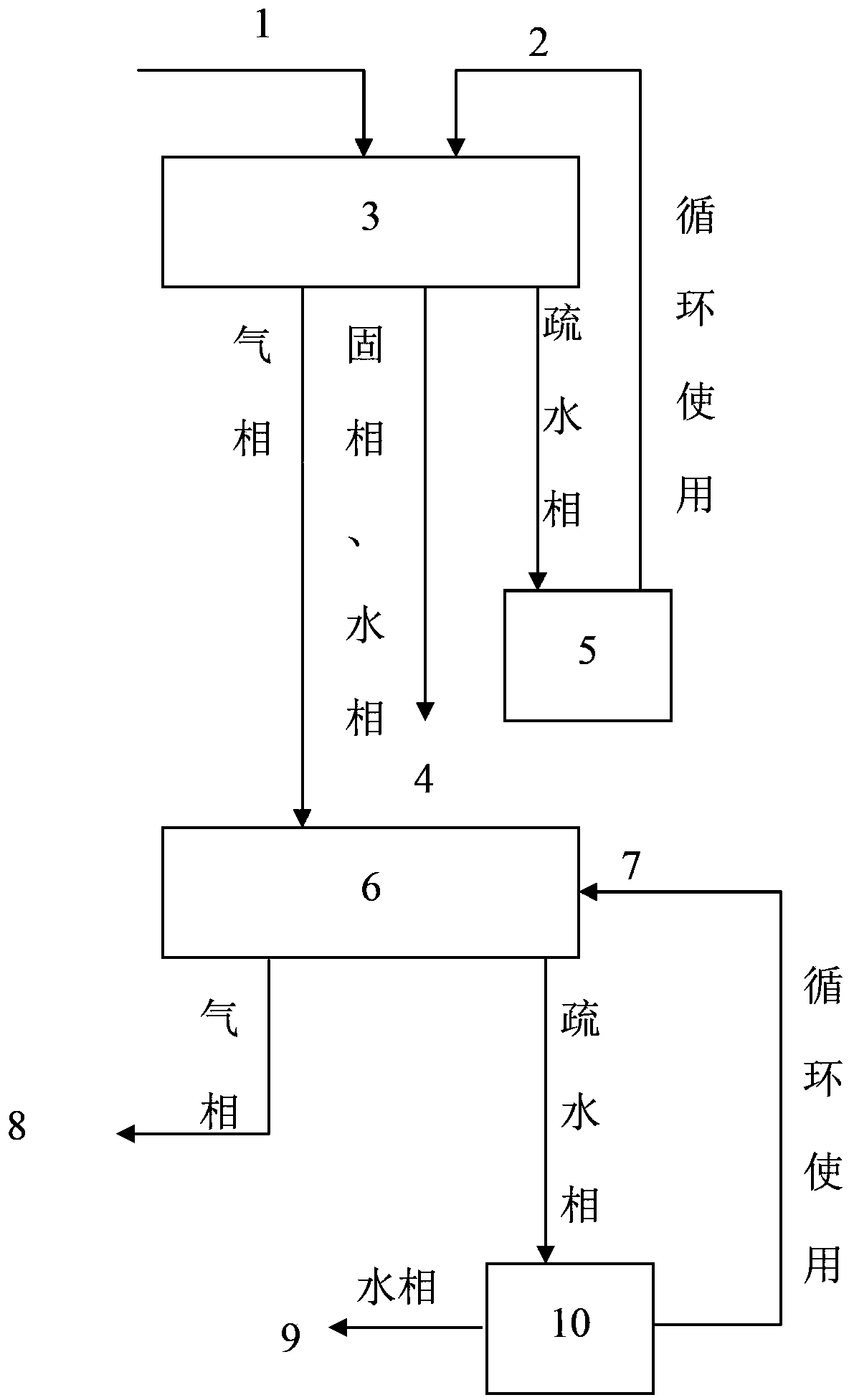
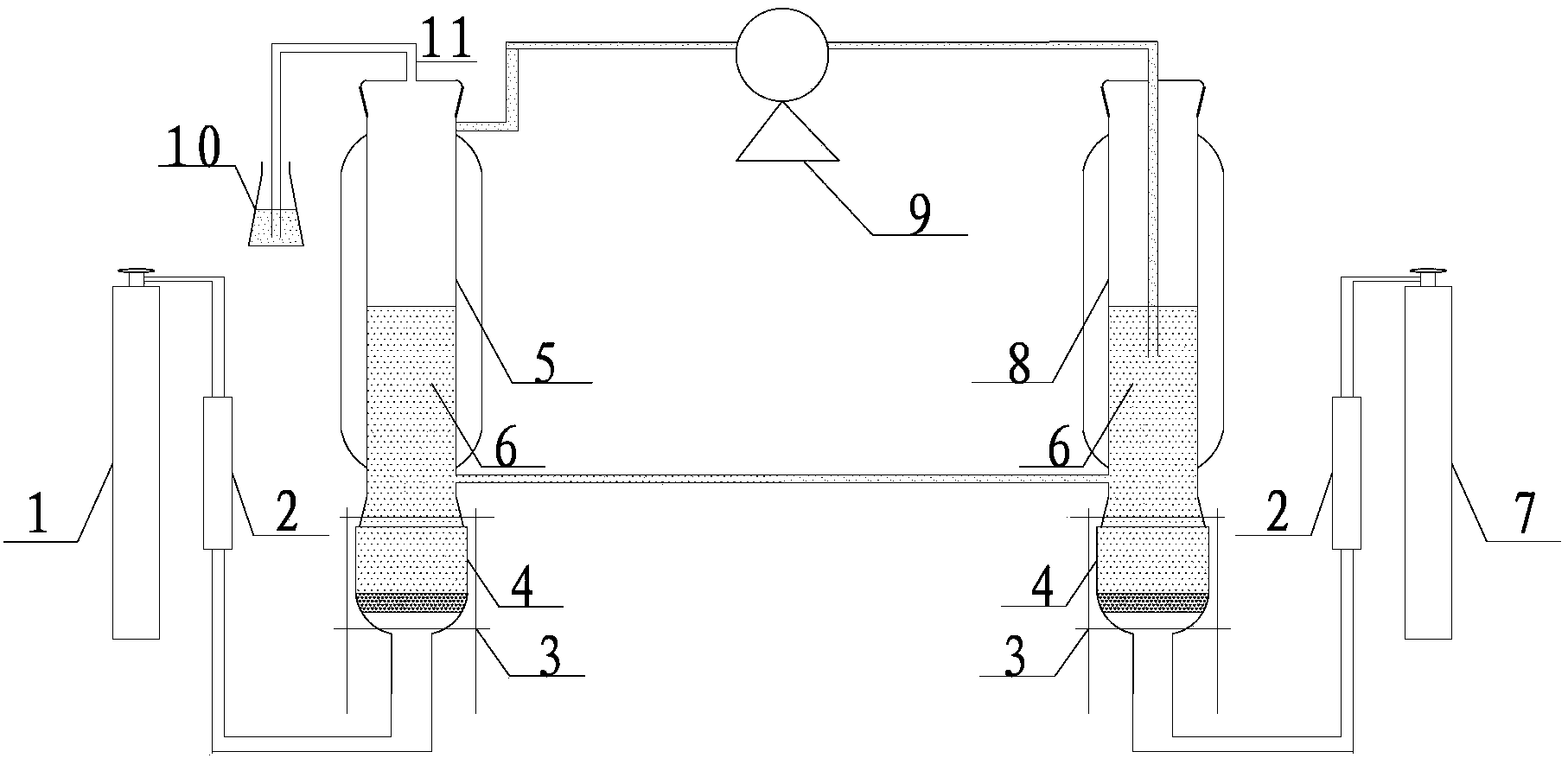
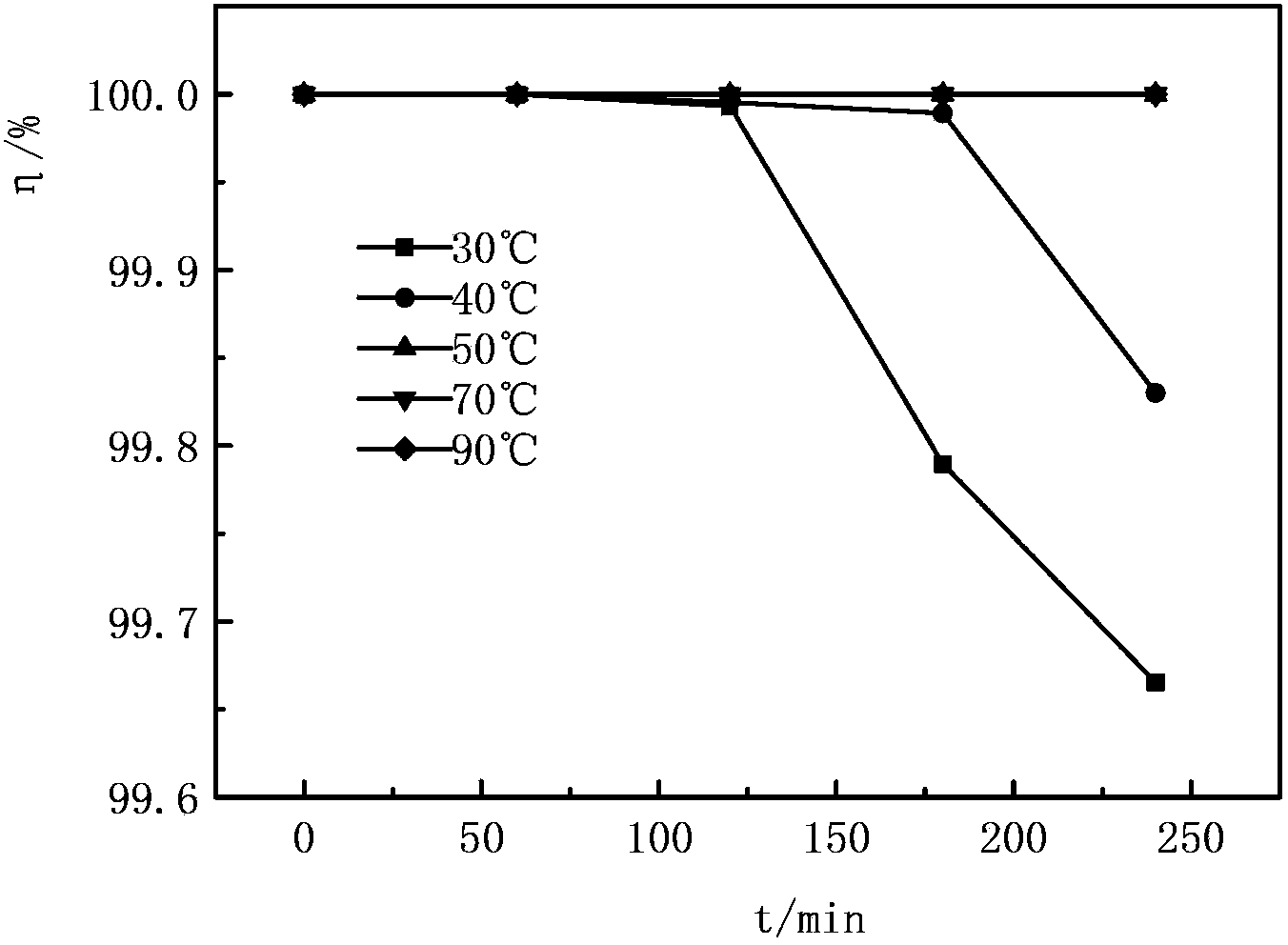
Image
Examples
Embodiment 1
[0046] Example 1: Preparation of iron-based ionic liquid (Fe-IL)
[0047] With 1-butyl-3-methylchloroimidazole (BmimCl) and ferric chloride hexahydrate (FeCl 3 ·6H 2 O) as the raw material, the molar ratio is 1:2 to synthesize iron-based ionic liquid (Fe-IL);
[0048] The mass fraction of iron ions in the iron-based ionic liquid measured by the HI83200 (C200) multi-parameter ion analyzer is 14.4%, and the mass fractions of C, H, and N in the iron-based ionic liquid measured by the Vario El element analyzer are respectively 26.2%, 4.3%, 8.6%, therefore, the chemical formula of iron-based ionic liquid can be calculated as C 8 H 15 N 2 FeCl 4 , Can also be recorded as [Bmim]FeCl 4 (The same below).
Embodiment 2
[0049] Example 2: Preparation of Fe-Pd-based ionic liquid (Fe-Pd-IL)
[0050] Mixing 1-butyl-3-methylchloroimidazole with a mass ratio of 49:1 and palladium chloride in an open natural environment at 80° C. to obtain a palladium-based ionic liquid;
[0051] According to GB / T23276-2009, the determination was carried out by the EDTA complexometric titration method with dimethylglyoxime precipitation. The mass fraction of palladium ions in the palladium-based ionic liquid was measured to be 1.2%. The palladium was measured with a Vario El type elemental analyzer. The mass fractions of C, H, and N in the base ionic liquid are 53.9%, 8.4%, and 15.7%, respectively. Therefore, the chemical formula of the palladium-based ionic liquid can be calculated as [BmimCl] 50 PdCl 2 ;
[0052] The iron-based ionic liquid prepared in Example 1 and the palladium-based ionic liquid were mixed thoroughly in an open natural environment at a mass ratio of 1000:1, and the Fe-Pd-based ionic liquid prepared wa...
Embodiment 3
[0053] Example 3: Preparation of Fe-Cu-based ionic liquid (Fe-Cu-IL)
[0054] Mixing 1-butyl-3-methylchloroimidazole with a mass ratio of 49:1 and copper chloride in an open natural environment at 80°C to prepare a copper-based ionic liquid;
[0055] The mass fraction of copper ions in the copper-based ionic liquid measured by the HI83200 (C200) multi-parameter ion analyzer was 0.7%, and the mass fraction of C, H, and N in the copper-based ionic liquid was measured by the Vario El element analyzer. It is 54.1%, 8.5%, 15.8%, so the chemical formula of the copper-based ionic liquid can be written as [BmimCl] 48 CuCl 2 ;
[0056] The iron-based ionic liquid prepared in Example 1 and the copper-based ionic liquid were mixed thoroughly in an open natural environment at a mass ratio of 1000:1, and the Fe-Cu-based ionic liquid prepared was denoted as Fe-Cu-IL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com