Blumea balsamifera controlled release preparation and preparation method thereof
A controlled-release preparation, the technology of Ainaxiang, which is applied in the direction of pharmaceutical formulas, medical preparations containing active ingredients, plant raw materials, etc., can solve the problems of reducing the frequency and dosage of daily dosage, reducing the daily dosage of patients, and achieving the reduction of medication The number of times, the maintenance time of promoting blood circulation and removing blood stasis, and the effect of maintaining blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
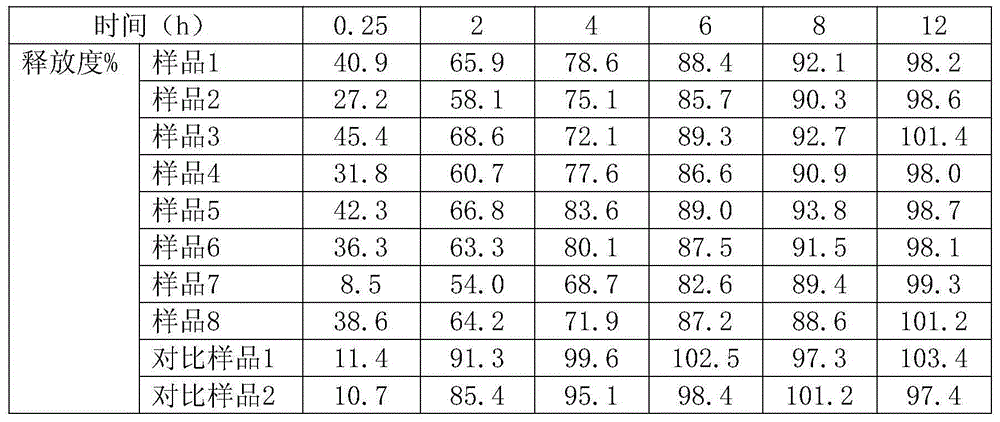
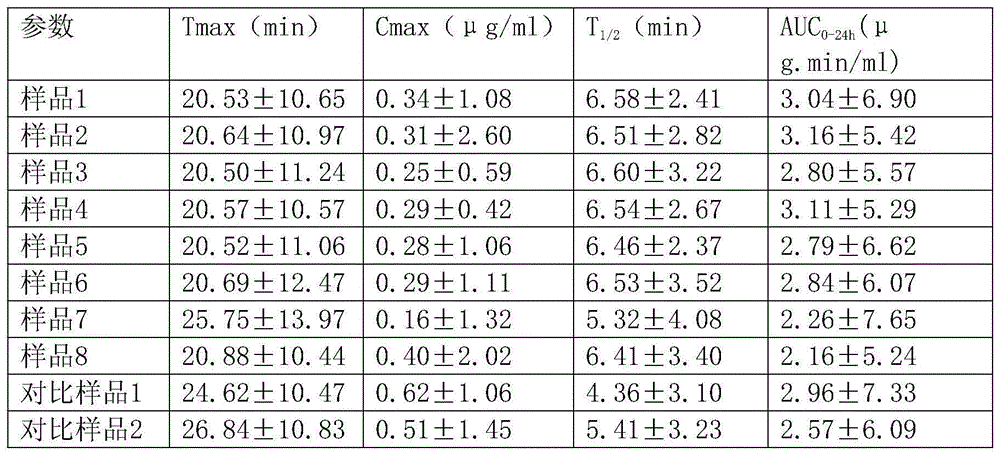
Examples
Embodiment 1
[0023] Prescription of controlled-release Diangui Ainaxiang pellets:
[0024] Pellets containing medicine: main drug - 440g of dry paste powder of Ainaxiang yunnanensis extract; filler - 70.7g of microcrystalline cellulose, 600035.3g of polyethylene glycol;
[0025] Isolation layer: coating material - HPMCE513.1g; anti-sticking agent - talcum powder 2.9g;
[0026] Controlled release layer: controlled release coating material - EudragitNE30D40.6g; porogen - povidone K301.9g; anti-sticking agent - talc powder 16.4g;
[0027] Prescription of quick-release Diangui Ainaxiang components:
[0028] Main drug - 220g of dry paste powder of Yunnan Gui Ainaxiang extract; filler - 164g of starch-lactose complex, 119g of pregelatinized starch; disintegrating agent - 22g of croscarmellose sodium, 11g of micropowdered silica gel ;Lubricant—magnesium stearate 1.6g;
[0029] Prepare the mixed prescription quantity of the preparation:
[0030] 495.4g of controlled-release Diangui Ainaxiang p...
Embodiment 2
[0041] Prescription of controlled-release Diangui Ainaxiang pellets:
[0042] Drug-containing pellets: main drug - Diangui Ainaxiang 462g; filler - microcrystalline cellulose 111.4g;
[0043] Isolation layer: coating material - HPMCE 513.8g; anti-sticking agent - talc powder 3.1g;
[0044] Controlled-release layer: controlled-release coating materials—EudragitRS30D41.8g, EudragitRL30D1.8g; anti-sticking agent—talc powder 17.2g; plasticizer—glyceryl triacetate 3.3g;
[0045] Prescription of quick-release Diangui Ainaxiang ingredients:
[0046] Main drug—Diangui Ainaxiang 198g; filler—starch lactose complex 113g, microcrystalline cellulose 142g; disintegrant—cross-linked carmellose sodium 20g, micropowder silica gel 10g; lubricant—— Magnesium stearate 1.5g;
[0047] Prepare the mixed prescription quantity of the preparation:
[0048] 484.5g of controlled-release Diangui Ainaxiang pellets and 484.5g of quick-release Diangui Ainaxiang components, a total of 969g, were compress...
Embodiment 3
[0058] Prescription of controlled-release Diangui Ainaxiang pellets:
[0059] Drug-containing pellets: main drug - Diangui Ainaxiang 360g; filler - lactose 51g, calcium hydrogen phosphate 26g;
[0060] Isolation layer: coating material - HPMCE 58.5g; anti-sticking agent - talcum powder 5.7g;
[0061] Controlled-release layer: controlled-release coating material - 23.7g of Sulis; porogen - 2.8g of lactose;
[0062] Prescription of quick-release Diangui Ainaxiang components:
[0063] Main drug - Diangui Ainaxiang 240g; filler - starch lactose complex 222g; disintegrant - crospovidone 20g, micropowder silica gel 19g; lubricant - magnesium stearate 2.5g;
[0064] Prepare the mixed prescription quantity of the preparation:
[0065] 477.7g of controlled-release Diangui Ainaxiang pellets and 503.5g of quick-release Diangui Ainaxiang components, a total of 981.2g, were compressed into 1000 tablets.
[0066] According to the specific preparation method of the above prescription:
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com