Electrolysis method for separating copper from cobalt and nickel in chloride ion ammoniacal system and application of products thereof
An electrolytic separation and chloride ion technology, which is applied in the material metallurgy of cobalt and nickel, and directly separates copper, can solve the problems of difficult large-scale industrial application, loss of nickel and cobalt, and large amount of washing water, and achieves good product consistency and low cost. , the effect of simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
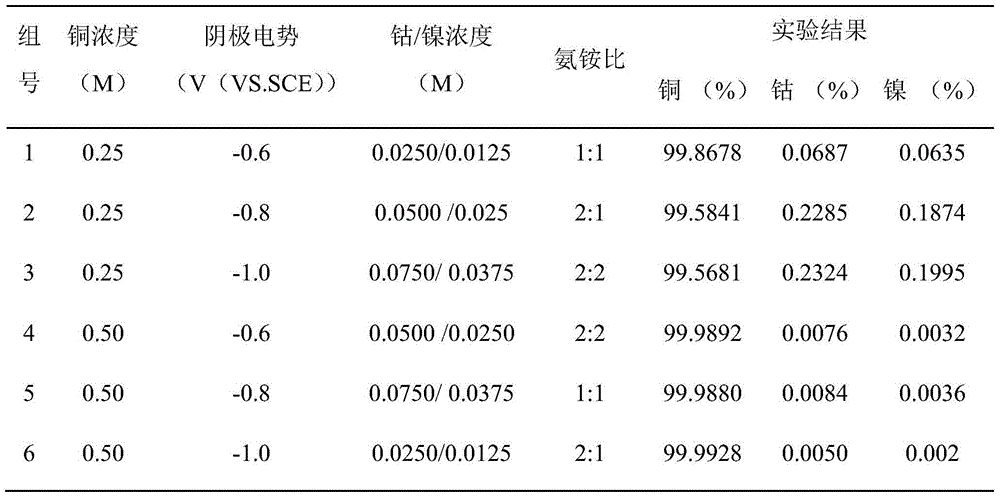
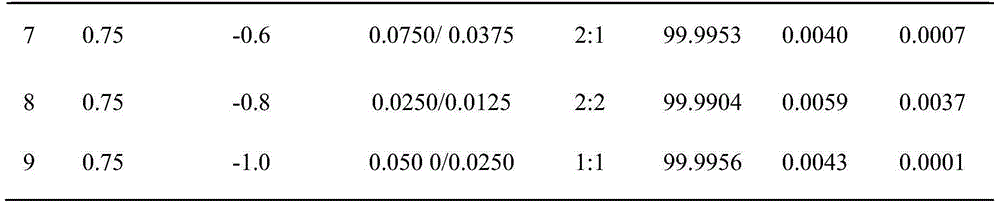
[0027] A method for electrolytically separating copper and cobalt-nickel in the chlorine ion ammoniacal system of the present invention, the method mainly adopts the electrolytic separation method, comprising the following steps:
[0028] (1) Preparation of electrolyte system: Add 21.31g of copper chloride, 2.95g of cobalt chloride and 1.49g of nickel chloride to the leaching solution of simulated ore samples containing copper, cobalt and nickel, and then add 18.52g of chlorine to the leaching solution Ammonium chloride and 29.22g of sodium chloride were mixed and dissolved, and finally 9.13ml of concentrated ammonia water was added to prepare a 500ml electrolyte system;
[0029] (2) Electrolytic separation: In the electrolyte system prepared above, graphite is used as the electrolytic anode, and stainless steel plate is used as the electrolytic cathode. The solution is degreased and then rinsed with distilled water before use; the electrolytic separation of the above electrol...
Embodiment 2
[0031] A method for electrolytically separating copper and cobalt-nickel in the chlorine ion ammoniacal system of the present invention, the method mainly adopts the electrolytic separation method, comprising the following steps:
[0032] (1) Preparation of electrolyte system: Add 21.31g of copper chloride, 5.90g of cobalt chloride and 2.98g of nickel chloride to the simulated ore sample leaching solution containing copper, cobalt and nickel, and then add 18.52g of chlorine to the leaching solution Ammonium chloride and 29.22g of sodium chloride were mixed and dissolved, and finally 18.26ml of concentrated ammonia water was added to prepare a 500ml electrolyte system;
[0033] (2) Electrolytic separation: In the electrolyte system prepared above, graphite is used as the electrolytic anode, and stainless steel plate is used as the electrolytic cathode. The solution is degreased and then rinsed with distilled water before use; the above electrolyte system is electrolytically sep...
Embodiment 3
[0035] A method for electrolytically separating copper and cobalt-nickel in the chlorine ion ammoniacal system of the present invention, the method mainly adopts the electrolytic separation method, comprising the following steps:
[0036] (1) Preparation of electrolyte system: Add 21.31g of copper chloride, 8.85g of cobalt chloride and 4.47g of nickel chloride to the leaching solution of simulated ore samples containing copper, cobalt and nickel, and then add 37.04g of chlorine to the leaching solution Ammonium chloride and 29.22g of sodium chloride were mixed and dissolved, and finally 18.26ml of concentrated ammonia water was added to prepare a 500ml electrolyte system;
[0037] (2) Electrolytic separation: In the electrolyte system prepared above, graphite is used as the electrolytic anode, and stainless steel plate is used as the electrolytic cathode. The solution is degreased and then rinsed with distilled water before use; the electrolytic separation of the above electro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| area | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com