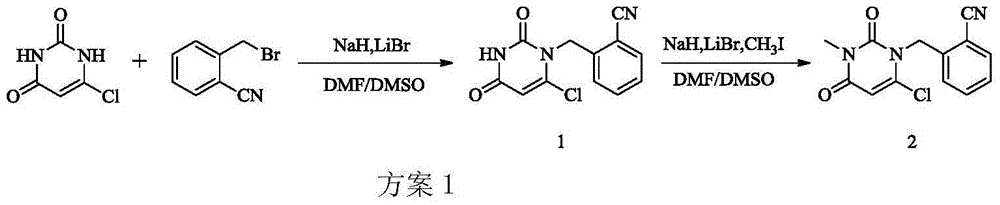
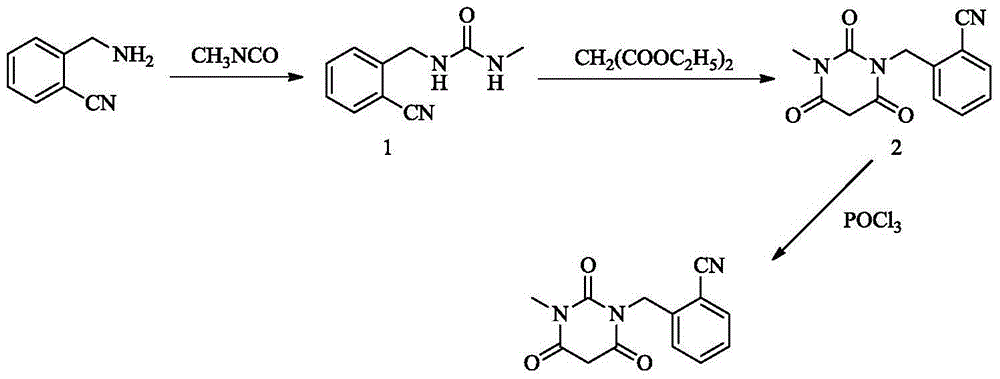
Synthesis method of Alogliptin intermediate
A synthetic method and an intermediate technology, which are applied in the field of synthesis of alogliptin intermediate 2-[-pyrimidinyl)methyl]benzonitrile, can solve problems such as toxicity, difficulty in obtaining, and difficulty in purification, and achieve synthesis Simple steps, easy-to-obtain raw materials, and low-cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Take 100g of methyl urea and 165mL of malonic acid, dissolve them in 300mL of acetic acid, stir and heat to 90°C. Take 540 mL of acetic anhydride and slowly drop it into the reaction solution. After the dropwise addition, keep warm and continue to stir the reaction. After the completion of the reaction was monitored by TLC, the reaction solution was cooled to room temperature and concentrated under reduced pressure to an oily state. Add 500 mL of ethanol and stir overnight at 0° C., a solid precipitates, and is filtered to obtain a yellow solid. The yellow solid was recrystallized with ethanol and decolorized by activated carbon to obtain 165 g of a light yellow solid with a yield of 86%, m.p. 3.03 (s, 3H, NCH3), 3.56 (s, 2H, CH2), 11.31 (s, 1H, NH).
Embodiment 2
[0029] Take 100 g of methyl urea and 237 g of diethyl malonate, dissolve them in a methanol solution of sodium methoxide (364 g), stir and heat to 90° C. for 12 hours. Cool down to room temperature, filter, and dissolve the filter cake in 500 mL of water. The aqueous solution was adjusted to pH 2-3 with concentrated hydrochloric acid, cooled to 0°C and stirred for 1 hour, a solid was precipitated, and a yellow solid was obtained by filtration. The yellow solid was recrystallized with ethanol and decolorized with activated carbon to obtain 126 g of a light yellow solid with a yield of 66%.
Embodiment 3
[0031] Take 100 g of methyl urea and 279 g of diisopropyl malonate, dissolve them in a methanol solution of sodium methoxide (364 g), stir and heat to 90° C. for 12 hours. Cool down to room temperature, filter, and dissolve the filter cake in 500 mL of water. The aqueous solution was adjusted to pH 2-3 with concentrated hydrochloric acid, cooled to 0°C and stirred for 1 hour, a solid was precipitated, and a yellow solid was obtained by filtration. The yellow solid was recrystallized with ethanol and decolorized by activated carbon to obtain 100 g of light yellow solid with a yield of 52%.
[0032] Synthesis of 3-methyl-6-chlorouracil
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com