Preparation method of roflumilast
A technology of roflumilast and difluoromethoxybenzoic acid, which is applied in the field of drug synthesis, can solve problems such as unfavorable industrial production, strong corrosion of equipment, and reduced production efficiency, so as to avoid high temperature and reduced pressure distillation and simple production conditions , The effect of increasing the reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
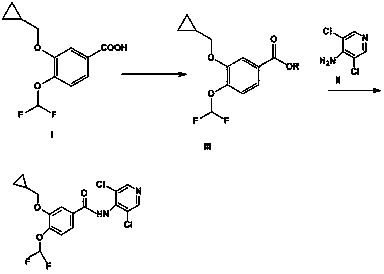
[0036] Dissolve 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (250g) in 2L of dichloromethane, then add 3,5-dichloro-4-aminopyridine (202g) and then Cool down to 0~20°C. Add 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid (330g) in batches, stir and lower the temperature by 10-20°C, and stir until the raw materials disappear.
[0037] Dissolve triethylamine (121.2g) in 0.2L of dichloromethane and add it dropwise to the above system. After the addition, continue to stir for 3 hours and then analyze the raw material 3-cyclopropylmethoxy-4-difluoromethoxy by TLC Benzoic acid basically disappeared. Suction filtration, wash the filter cake with a small amount of dichloromethane, then place the filtrate at 0~5°C for 12 hours, a small amount of white precipitate precipitates, filter, evaporate the filtrate to dryness to obtain 206g of the final product Roflumilast, and collect The yield is 85.6%, and the purity is 98.9%.
Embodiment 3
[0039]Dissolve 2,4-dimethoxy-6-chloro-1,3,5-s-triazine (5.25kg) in 3L of dichloromethane, and 3-cyclopropylmethoxy-4-difluoromethane Add oxybenzoic acid (6.6kg) to the above dichloromethane solution, stir and cool down to 0~10°C, add pyridine (2.4kg) dropwise to the above system, continue stirring for 3 hours after the addition, and analyze 3-cyclopropane by TLC The methoxy-4-difluoromethoxybenzoic acid basically disappeared.
[0040] Dissolve 3,5-dichloro-4-aminopyridine (4.07kg) in 2L of dichloromethane and add it dropwise to the reaction solution. Stirring is continued for 2 hours after the addition, and the intermediate active ester disappears according to TLC analysis. Suction filtration, the filtrate once with saturated NaHCO 3 solution, saturated NaCl solution, anhydrous Na 2 SO 4 Drying, suction filtration, and reduced pressure below 45°C yielded 6.432 kg of the final product, roflumilast, with a yield of 92.5% and a purity of 99.3%.
Embodiment 4
[0042] 1-Hydroxybenzotriazole (160.6 g) was dissolved in 2 L of dichloromethane, and 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid (258 g) was added to the above dichloromethane In the solution, stir and cool down to -10~0°C, add N-methylmorpholine (121.2g) dropwise to the above system, continue stirring for 3 hours after the addition, TLC analysis of the raw material 3-cyclopropylmethoxy-4 - Difluoromethoxybenzoic acid essentially disappeared.
[0043] 3,5-dichloro-4-aminopyridine (164 g) was added dropwise to the reaction solution, and stirring was continued for 2 h after addition, and the reaction was complete by TLC analysis. Suction filtration, the filtrate was successively washed with saturated NaHCO 3 solution, saturated NaCl solution, anhydrous Na 2 SO 4 Dry, filter with suction, and reduce pressure below 45°C to obtain the final product Roflumilast 260.5. Yield 92.0%, purity 99.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com