Lithium manganese phosphate nano-ellipsoids and preparation method thereof
A lithium manganese phosphate, nanotechnology, applied in nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of low cost, slow lithium ion diffusion, poor conductivity, etc., achieve low cost, improve high current charging and discharging Stable performance and quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
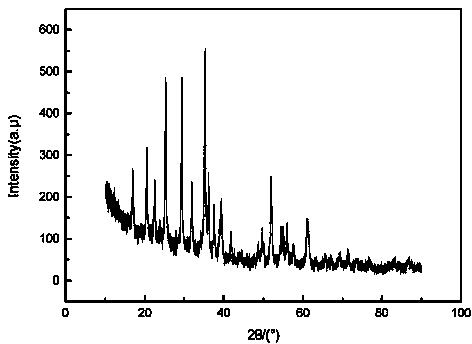
Image
Examples
example 1
[0023] 1) Weigh 1.470g of manganese acetate, dissolve it in 15 ml of ethylene glycol, stir for 10 minutes, then weigh 0.2000 g of ascorbic acid and dissolve it in the above solution, and stir until fully dissolved to obtain a concentration of manganese acetate of 0.4 mol / L and a concentration of ascorbic acid Solution A of 0.076 mol / L;
[0024] 2) Dissolve 0.588g of phosphoric acid and 0.612g of lithium acetate in 15ml of ethylene glycol and stir for 30 minutes to form a suspension B with a phosphoric acid concentration of 0.4 mol / L and a lithium acetate concentration of 0.4 mol / L;
[0025] 3) Add the suspension B prepared in step 2) dropwise to solution A in step 1) under stirring to form emulsion C, so that the molar ratio of Li, Mn, and P in emulsion C is 1 :1:1;
[0026] 4) Transfer the emulsion C in step 3) to an autoclave with a liner volume of 50ml, add 5 ml of ethylene glycol solution containing 0.112 g KOH, stir well, and then adjust its volume to 40ml with ethylene ...
example 2
[0030] 1) Weigh 2.45g of manganese acetate, dissolve it in 15 ml of ethylene glycol, stir for 60 minutes, then weigh 0.400 g of ascorbic acid and dissolve it in the above solution, and stir until fully dissolved to obtain manganese acetate concentration of 0.67 mol / L, ascorbic acid Solution A with a concentration of 0.152 mol / L;
[0031] 2) Dissolve 0.98 g of phosphoric acid and 2.04 g of lithium acetate in 15 ml of ethylene glycol and stir for 60 minutes to form a suspension B with a concentration of phosphoric acid of 0.67 mol / L and a concentration of lithium acetate of 1.33 mol / L;
[0032] 3) Add the suspension B prepared in step 2) dropwise to solution A in step 1) under stirring to form emulsion C, so that the molar ratio of Li, Mn, and P in emulsion C is 3 :1:1;
[0033] 4) Transfer the emulsion C in step 3) to an autoclave with a liner volume of 60ml, add 6 ml of ethylene glycol solution containing 0.224 g KOH, stir well, and then adjust its volume to 40ml with ethylen...
example 3
[0036] 1) Weigh 2.94g of manganese acetate, dissolve it in 15 ml of ethylene glycol, stir for 120 minutes, then weigh 0.240 g of ascorbic acid and dissolve it in the above solution, stir until fully dissolved, and obtain manganese acetate concentration of 0.80 mol / L, ascorbic acid Solution A with a concentration of 0.091 mol / L;
[0037] 2) Dissolve 1.176 g of phosphoric acid and 1.224 g of lithium acetate in 15 ml of ethylene glycol and stir for 120 minutes to form a suspension B with a phosphoric acid concentration of 0.80 mol / L and a lithium acetate concentration of 0.80 mol / L;
[0038] 3) Add the suspension B prepared in step 2) dropwise to solution A in step 1) under stirring to form emulsion C, so that the molar ratio of Li, Mn, and P in emulsion C is 1 :1:1;
[0039] 4) Transfer the emulsion C in step 3) to an autoclave with a liner volume of 55ml, add 5 ml of ethylene glycol solution containing 0.112 g KOH, stir well, and then adjust its volume to 40ml with ethylene gl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com