Lithium single ionic conductive microporous electrolyte membrane and preparation method thereof
An electrolyte membrane and single-ion technology, applied in the manufacture of electrolyte batteries, non-aqueous electrolytes, solid electrolytes, etc., can solve problems such as low ion conductivity, loss of migration ability, reduced lithium-ion battery capacity, cycle performance, and energy efficiency , to achieve the effect of simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
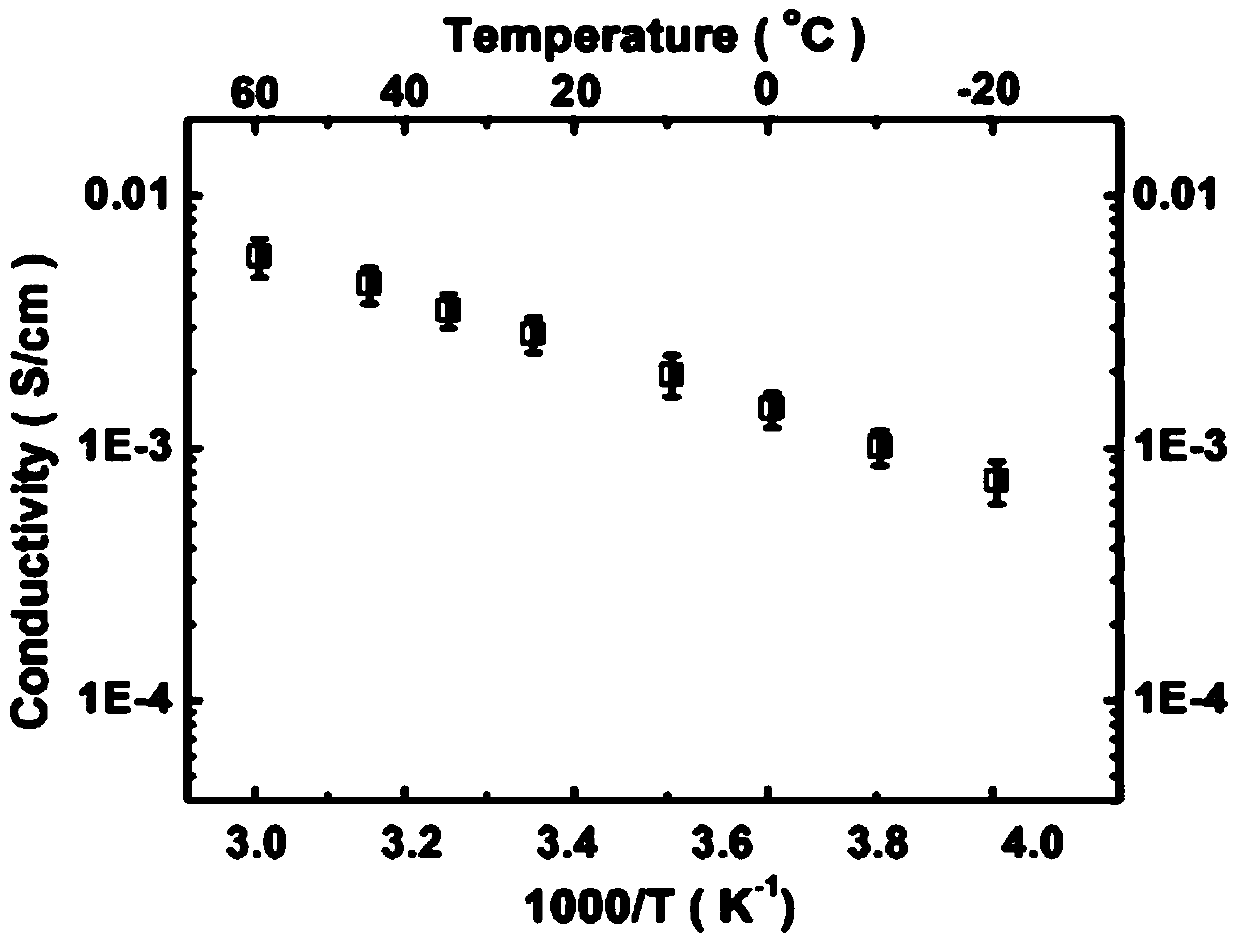
Image
Examples
Embodiment 1
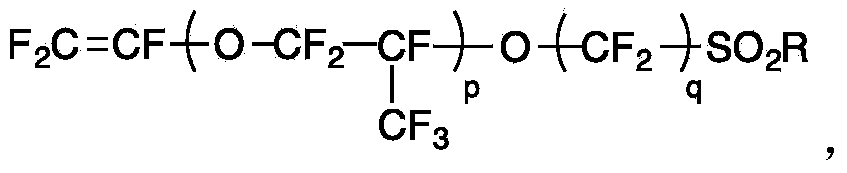
[0042] The polyphenylene ether polymer whose side group is lithium fluorosulfonate group has the following chemical structure:
[0043]
[0044] The preparation process of the polymer: add hydroquinone (10g), 4,4'-diphenol (17g), potassium carbonate (65g), anhydrous dimethyl Acetamide (450mL), toluene (180mL). The mixture was heated to reflux for 3 hours and the toluene / water azeotrope was removed with a trap. Sodium 2-(2',3',5',6'-tetrafluorophenoxy)tetrafluoroethanesulfonate (68 g) was then added to the flask and the reaction was maintained at 160°C for 12 hours. After the reaction was cooled to room temperature, the reaction mixture was precipitated in water, and the precipitate was collected by filtration and washed well with water. The obtained polymer was converted from the sodium salt form to the lithium salt by immersion in a 10 mol / L lithium triflate aqueous solution at 60° C. for 15 hours. After filtering and fully washing with water, the obtained polyphenylene...
Embodiment 2
[0051] The polyphenylene ether polymer whose side group is lithium fluorosulfonate group has the following chemical structure:
[0052]
[0053] The preparation process of the polymer: add hydroquinone (10 g), potassium carbonate (33 g), anhydrous dimethylacetamide (280 mL) and toluene (120 mL) into a three-necked flask protected by an argon atmosphere. The mixture was heated to reflux for 3 hours and the toluene / water azeotrope was removed with a trap. Sodium 2-(2',3',5',6'-tetrafluorophenoxy)tetrafluoroethanesulfonate (38 g) was then added to the flask and the reaction was maintained at 160°C for 12 hours. After the reaction was cooled to room temperature, the reaction mixture was precipitated in water, and the precipitate was collected by filtration and washed well with water. The obtained polymer was impregnated with 10 mol / L LiSO at 60 °C 3 CF 3 It was converted from the sodium salt form to the lithium salt in aqueous solution for 15 hours. After filtering and full...
Embodiment 3
[0057] Take by weighing 60 grams of Nafion fluorine-containing sulfonic acid resin, 40 grams of polyethylene glycol (average molecular weight 1000); Nafion fluorine-containing sulfonic acid resin is placed in the LiOH saturated aqueous solution of 5mol / L and its fluorine-containing sulfonic acid conversion is carried out by ion exchange After forming fluorine-containing lithium sulfonate, it was dissolved in 1500 grams of N,N-dimethylformamide with polyethylene glycol; the prepared solution was spread on a clean glass plate by solution casting method, and allowed to The solvent evaporates to form a film; the glass plate and the film are immersed in a room temperature alcohol-water mixed solution (30% volume of methanol) for 60 minutes to separate the film from the glass plate, and the film is soaked in new 60°C deionized water for 560 minutes, and then again Soak in new deionized water at room temperature for 220 minutes, extract the polyethylene glycol in the membrane to obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| tensile strength | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com