Lansoprazole enteric-coated tablet and preparation method thereof
A technology for lansoprazole enteric and lansoprazole, which is applied in the field of lansoprazole enteric-coated tablets and the preparation thereof, can solve the problems of unstable process quality control and the like, achieves improvement of oral bioavailability and reduction of batch-to-batch The effect of different release and rapid disintegration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
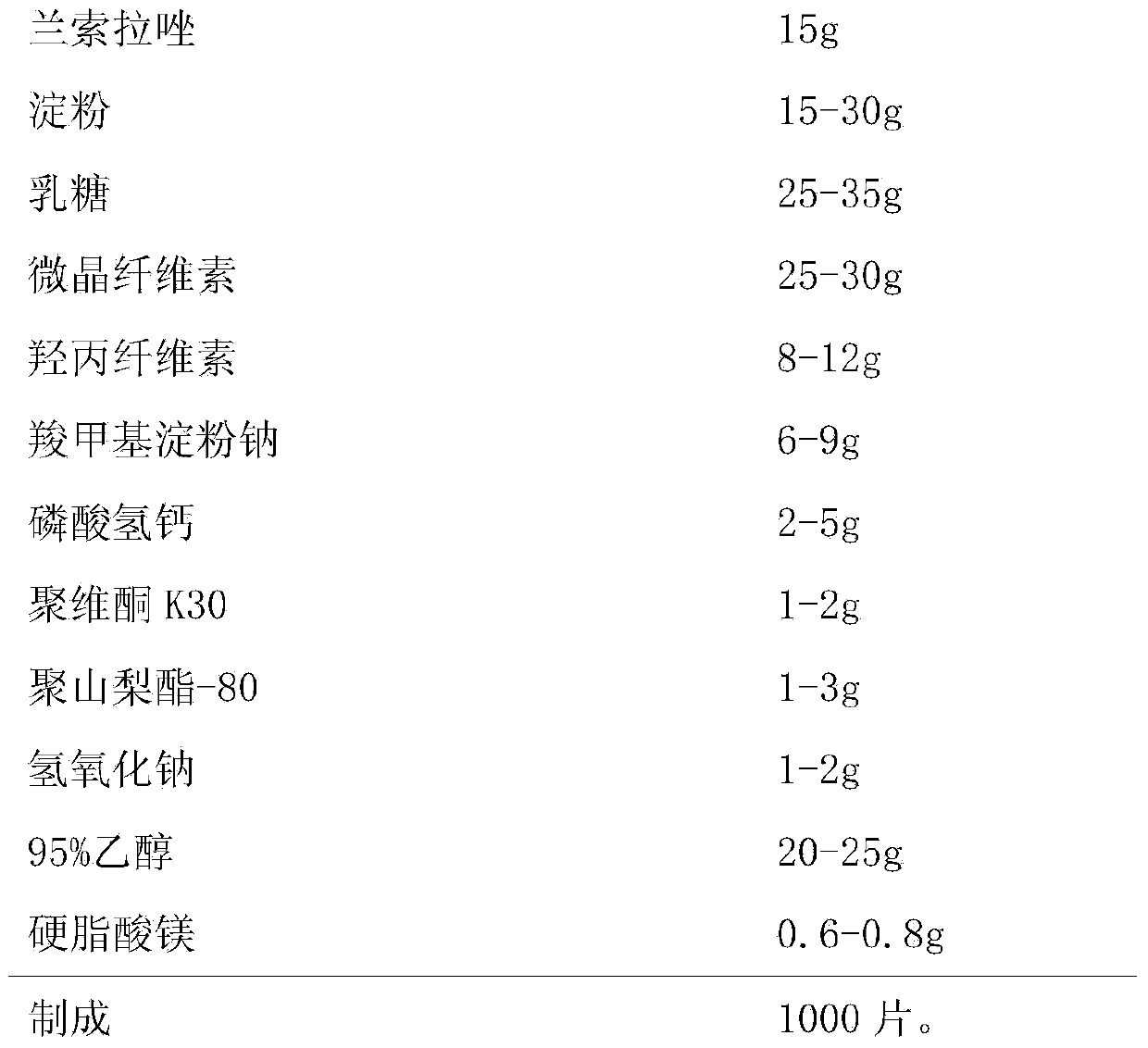
[0030] Embodiment 1, a kind of Lansoprazole enteric-coated tablet, the composition of every 1000 is as follows,
[0031] Chip:
[0032]
[0033] Isolation layer coating: hydroxypropyl methylcellulose ethanol solution (containing 1% HPMC, 3% talc, 3% PEG 6000, 1% Tween 280, 1% diethyl phthalate, 80% 95% ethanol );
[0034] Enteric layer coating: polyacrylic acid resin EudragitL100-55 aqueous suspension (containing 13% EudragitL100-55, 0.1% sodium hydroxide, 2% PEG6000, 3.4% talc, 1.5% titanium dioxide)
[0035] Above-mentioned lansoprazole enteric-coated tablet preparation method is:
[0036] (1) Take by weighing a sufficient amount of starch, lactose, microcrystalline cellulose, hydroxypropyl cellulose, calcium hydrogen phosphate and 6g sodium carboxymethyl starch according to the prescription quantity and mix;
[0037] (2) measure 95% ethanol, add the sodium hydroxide of prescription quantity, stir and make dissolving; Add the povidone K30 of prescription quantity, stir...
Embodiment 2
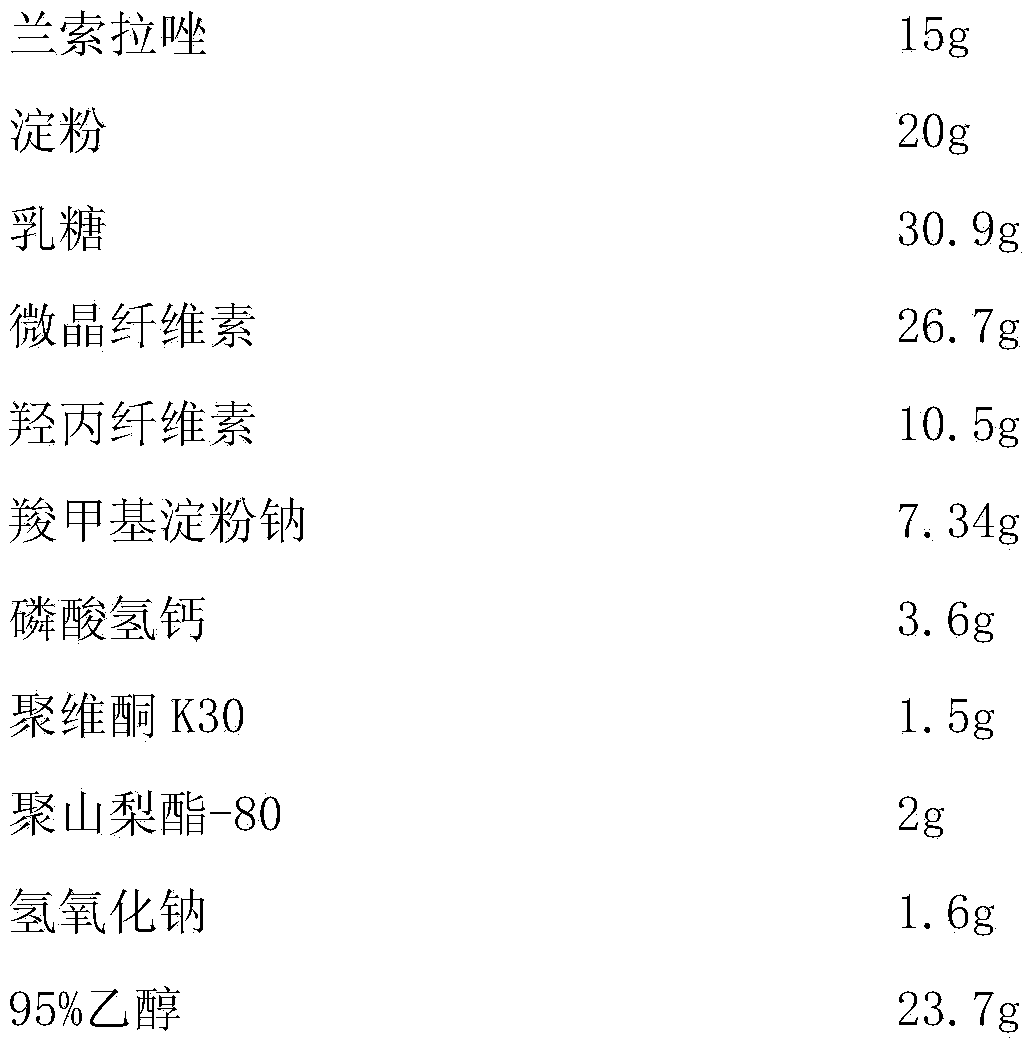
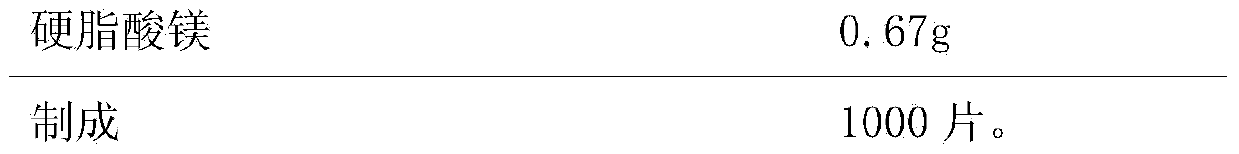
[0044] Embodiment 2, a kind of Lansoprazole enteric-coated tablet, the composition of every 1000 is as follows,
[0045] Chip:
[0046]
[0047] Isolation layer coating: hypromellose ethanol solution (containing 1% HPMC, 3% talc, 3% PEG 6000, 1% Tween 280, 1% diethyl phthalate, 80% 95% ethanol );
[0048] Enteric layer coating: polyacrylic acid resin EudragitL100-55 aqueous suspension (containing 13% EudragitL100-55, 0.1% sodium hydroxide, 2% PEG6000, 3.4% talc, 1.5% titanium dioxide)
[0049] Above-mentioned lansoprazole enteric-coated tablet preparation method is:
[0050] (1) Take by weighing a sufficient amount of starch, lactose, microcrystalline cellulose, hydroxypropyl cellulose, calcium hydrogen phosphate and 4.8g sodium carboxymethyl starch according to the prescription and mix;
[0051] (2) measure 95% ethanol, add the sodium hydroxide of prescription quantity, stir and make dissolving; Add the povidone K30 of prescription quantity, stir and make dissolving; Ad...
Embodiment 3
[0058] Embodiment 3, a kind of Lansoprazole enteric-coated tablet, the composition of every 1000 is as follows,
[0059] Chip:
[0060]
[0061]
[0062] Isolation layer coating: hydroxypropyl methylcellulose ethanol solution (containing 1% HPMC, 3% talc, 3% PEG 6000, 1% Tween 280, 1% diethyl phthalate, 80% 95% ethanol );
[0063] Enteric layer coating: polyacrylic acid resin EudragitL100-55 aqueous suspension (containing 13% EudragitL100-55, 0.1% sodium hydroxide, 2% PEG6000, 3.4% talc, 1.5% titanium dioxide)
[0064] Above-mentioned lansoprazole enteric-coated tablet preparation method is:
[0065] (1) Take by weighing a sufficient amount of starch, lactose, microcrystalline cellulose, hydroxypropyl cellulose, calcium hydrogen phosphate and 7.38g sodium carboxymethyl starch according to the prescription and mix;
[0066] (2) measure 95% ethanol, add the sodium hydroxide of prescription quantity, stir and make dissolving; Add the povidone K30 of prescription quantity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com