Method for production of fuel ethanol from acetic acid
A technology for fuel ethanol and ethyl acetate, which can be applied in chemical instruments and methods, preparation of organic compounds, separation/purification of hydroxyl compounds, etc., and can solve the problems of increased size, energy consumption, low corrosion strength, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
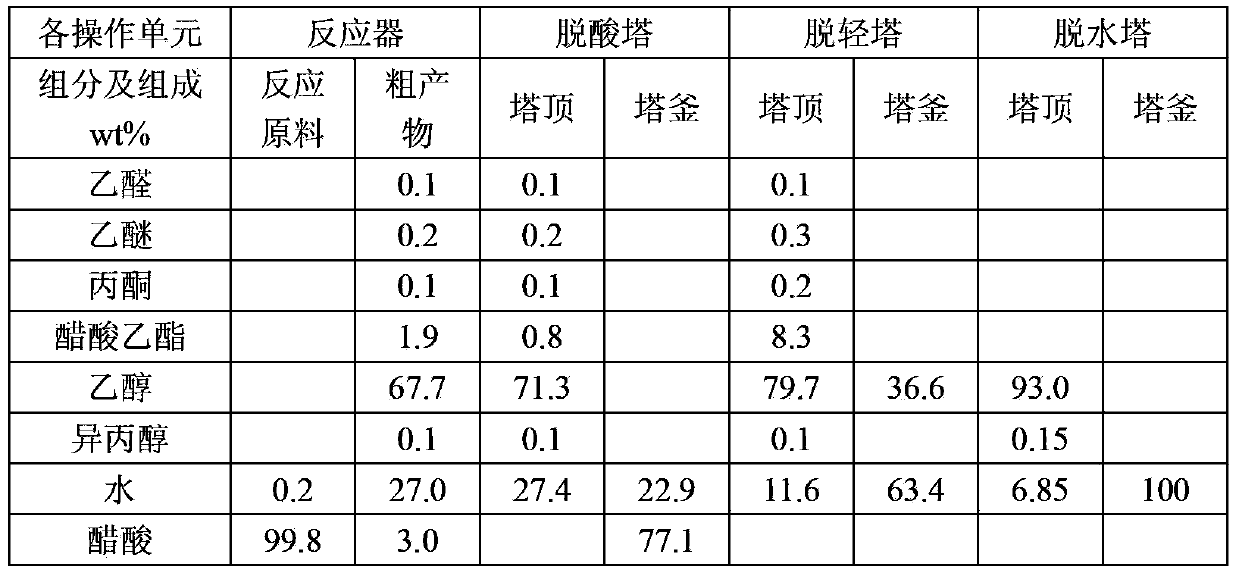
Embodiment 1
[0048] In this example, the catalyst for acetic acid hydrogenation is the acetic acid hydrogenation catalyst produced by Beijing Research Institute of Chemical Industry, the brand is BC-E-20, and the catalyst contains: (1) cobalt, wherein the cobalt metal content accounts for 30wt% of the total weight of the catalyst ; (2) molybdenum and chromium, respectively account for 2wt% of the total weight of the catalyst; the balance is silicon oxide. The catalyst is prepared by co-precipitation.
[0049] The method for producing fuel ethanol by acetic acid comprises the following steps:
[0050] (1) Hydrogenation: Preheat the acetic acid raw material and hydrogen, and then make it pass through a reactor equipped with a hydrogenation catalyst to hydrogenate acetic acid into crude ethanol product; the process conditions of acetic acid hydrogenation are: the liquid phase volume of acetic acid is empty The speed is 0.5h -1 , the molar ratio of hydrogen to acetic acid is 20:1, the reacti...
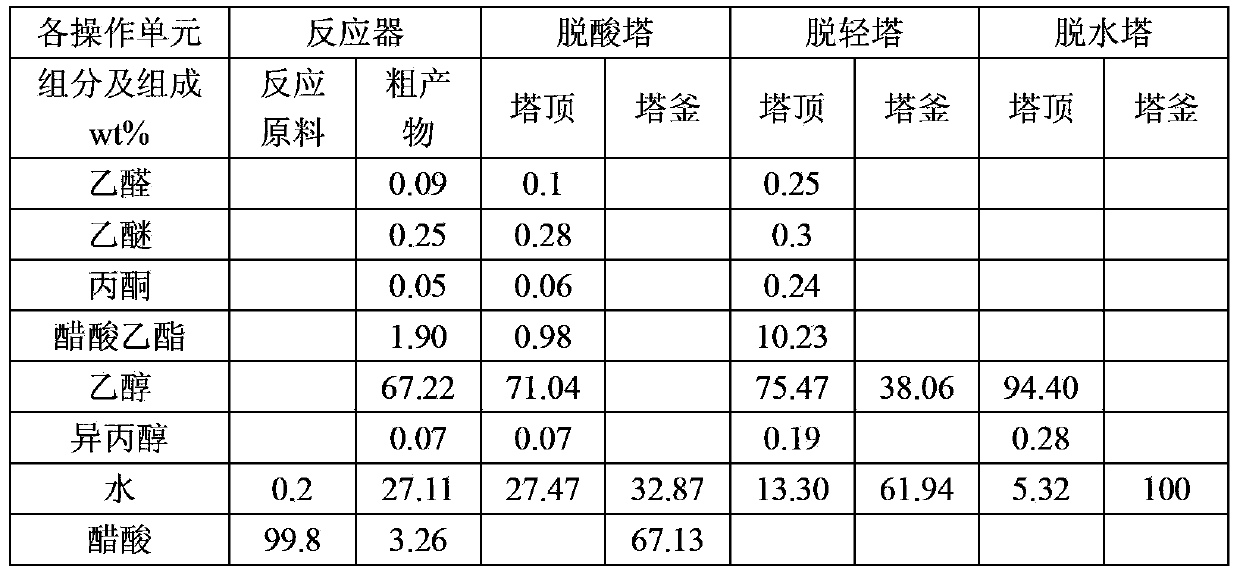
Embodiment 2
[0058] In this example, the acetic acid hydrogenation catalyst is the acetic acid hydrogenation catalyst produced by Beijing Research Institute of Chemical Industry, the brand is BC-E-20, and the catalyst contains: (1) cobalt, wherein the cobalt metal content accounts for 30wt% of the total weight of the catalyst ; (2) molybdenum and chromium, respectively account for 2wt% of the total catalyst weight; the balance is silicon oxide. The catalyst is prepared by co-precipitation.
[0059] The method for producing ethanol by acetic acid comprises the following steps:
[0060](1) Hydrogenation: Preheat the acetic acid raw material and hydrogen, and then make it pass through a reactor equipped with a hydrogenation catalyst to hydrogenate acetic acid into crude ethanol product; the process conditions of acetic acid hydrogenation are: the liquid phase volume of acetic acid is empty The speed is 0.75h -1 , the molar ratio of hydrogen to acetic acid is 15:1, the reaction temperature i...
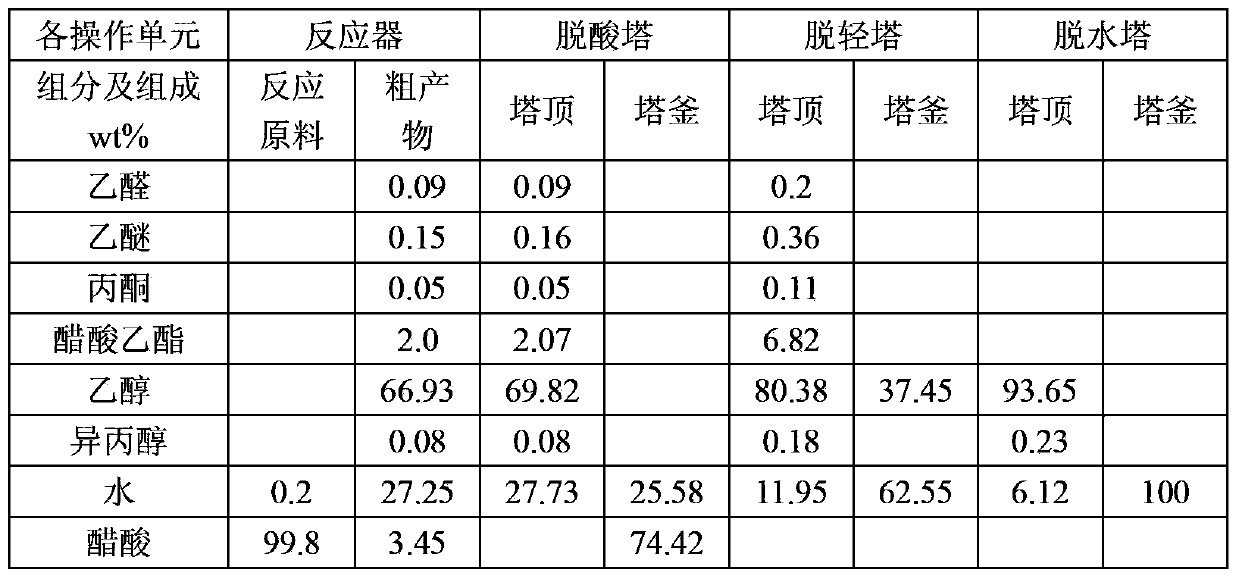
Embodiment 3
[0068] In this example, the catalyst for acetic acid hydrogenation is the acetic acid hydrogenation catalyst produced by Beijing Research Institute of Chemical Industry. The catalyst contains: (1) cobalt, wherein the cobalt metal content accounts for 35wt% of the total weight of the catalyst; (2) barium and silver, Account for 1.5wt% of the total weight of the catalyst; the balance is silicon oxide. The catalyst is prepared by a homogeneous deposition method.
[0069] The method for producing ethanol by acetic acid comprises the following steps:
[0070] (1) Hydrogenation: Preheat the acetic acid raw material and hydrogen, and then make it pass through a reactor equipped with a hydrogenation catalyst to hydrogenate acetic acid into crude ethanol product; the process conditions of acetic acid hydrogenation are: the liquid phase volume of acetic acid is empty The speed is 0.5h -1 , the molar ratio of hydrogen to acetic acid is 17:1, the reaction temperature is 275°C, and the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com