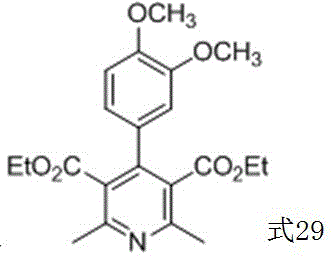
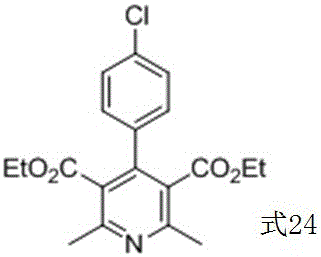
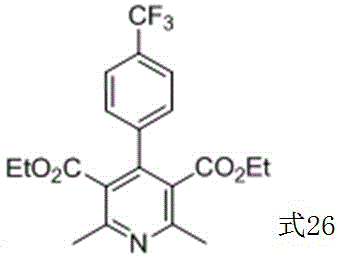
Method for preparing corresponding pyridine compound with 1,4-dihydropyridine compound
A technology of dihydropyridine and compounds, which is applied in the field of preparation of pyridine compounds, can solve the problems of low atom utilization, low efficiency, large pollutants, etc., achieve high practical production significance and industrial application value, simple photosensitive oxidation operation, The effect of reducing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1 Preparation of compound 1 as shown in formula 18
[0020]
[0021] Take a reaction test tube, add 34.6 mg Nifedipine (Nifedipine) 2,6-dimethyl-4-o-nitrophenyl-1,4-dihydropyridine-3,5-dicarbonate methyl ester, 1.13 mg of tetra-n-butylammonium salt TBA-eosinY of organic photosensitizer Eosin Y, 27.6 mg of potassium carbonate, 5.5 mL of mixed solvent of water and ethanol (ethanol and water are composed at a volume ratio of 10:1), at room temperature After reacting for 12 hours, spin-drying under vacuum, and passing through the column with petroleum ether and acetone, the yield of brownish-yellow oily liquid was 73.3%.
[0022] 1 H NMR: (400MHz, CDCl 3 )δ = 7.74-7.70 (m, 1H), 7.54-7.51 (m, 1H), 7.47-7.43 (m, 1H), 6.56 (d, J=8.0Hz, 1H), 3.39 (s, 6H), 2.68 (s, 6H) ppm. 13 C NMR: (400 MHz, CDCl 3 ):δ =167.3, 161.5, 156.2, 135.1, 130.9, 130.5, 129.0, 128.8,127.6, 65.6, 52.1, 29.7, 23.1, 19.2, 14.0 ppm. MS(70eV): m / z(%) : 3084.1( )[M + ], HRMS m / z (ESI) ca...
Embodiment 2
[0023] Embodiment 2 Preparation of compound 2 as shown in formula 19
[0024]
[0025] Take a reaction test tube, add 38.3 mg Felodipine (Felodipine) 4-(2,3-dichlorophenyl)-2,6-dimethyl-1,4-dihydropyridine-3-ethyl carbonate Ester-5-methyl carbonate, 1.13 mg of tetra-n-butylammonium salt TBA-eosinY of organic photosensitizer eosin Y, 27.6 mg of potassium carbonate, 5.5 mL of mixed solvent of water and ethanol (ethanol and water in a ratio of 10:1 volume ratio composition), reacted at room temperature for 12 hours, spin-dried in vacuum, and passed through the column with petroleum ether and acetone to obtain a light yellow oily liquid with a yield of 83.1%.
[0026] 1 H NMR: (400MHz, CDCl 3 ) δ =7.48 (dd, J = 6.8, 1.2Hz, 1H), 7.24-7.20 (t, J = 8.0Hz, 1H), 7.07 (dd, J = 6.0, 1.6Hz, 1H), 4.06-4.00 (m, 2H), 3.56 (s,3H), 2.66 (d, J = 5.6Hz, 6H), 0.97-0.93 (t, J = 7.2Hz, 3H) ppm. 13 C NMR: (400MHz, CDCl 3): δ =167.3, 166.7, 156.9, 156.7, 144.2, 137.7, 133.1, 131.5,130....
Embodiment 3
[0027] Preparation of compound 3 as shown in formula 20 in embodiment 3
[0028]
[0029] Take a reaction test tube, add 8 mg Nimodipine (Nimodipine) 2,6-dimethyl-4-m-nitrophenyl-1,4-dihydropyridine-3-isopropyl carbonate-5- 2-methoxyethyl carbonate, 0.2 mg tetra-n-butylammonium salt TBA-eosinY of organic photosensitizer eosin Y, 15.7 μL of 1,8-diazabicycloundec-7-ene, 2 mL of benzotrifluoride was reacted at room temperature for 12 hours, spin-dried in vacuo, and passed through the column with petroleum ether and acetone to obtain a colorless liquid with a yield of 78.4%.
[0030] 1 H NMR: (400MHz, CDCl 3 ) δ = 8.28-8.25 (m, 1H), 8.20-8.19(t, J = 1.6Hz,1H), 7.64-7.56 (m, 2H), 5.00-4.90 (m, 1H), 4.13 (s, 2H), 3.35 (s, 2H), 3.24(s, 3H), 2.65(d, J = 6.4Hz, 6H), 1.02(d, J = 6.4Hz, 6H) ppm. 13 C NMR: (400MHz, CDCl 3 ): δ=167.0, 166.5, 156.0, 155.9, 147.9, 137.9, 134.4, 129.2, 127.1, 123.5, 123.4, 69.8, 69.7, 64.4, 58.7, 22.9, 22.8, 21:3 ppm (MS) %): 416.1(584) [M + ], ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com