Phenolphthalein type copolyimide and preparation method thereof
A phenolphthalein type copolyimide and a technology of the copolyimide, which are applied in the field of phenolphthalein type copolyimide and its preparation, can solve the problems of high cost, complicated process, easy hydrolysis and the like, and achieve good heat resistance and solubility , The effect of reducing production cost and improving processability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
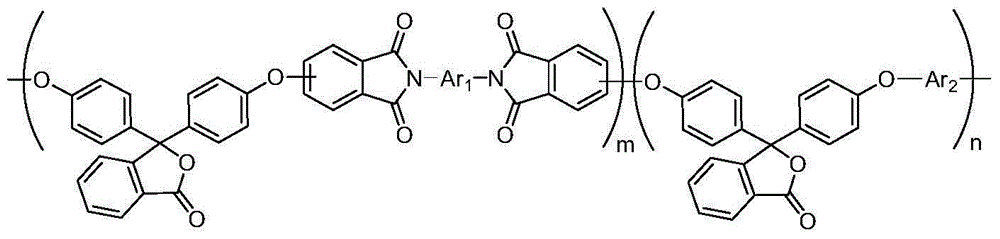
[0051] In this embodiment, the copolyimide has the following structural formula:
[0052]
[0053] The reaction scheme of above-mentioned copolyimide is as follows:
[0054]
[0055] Wherein, both m and n are integers greater than or equal to 1.
[0056] Its preparation method is as follows:
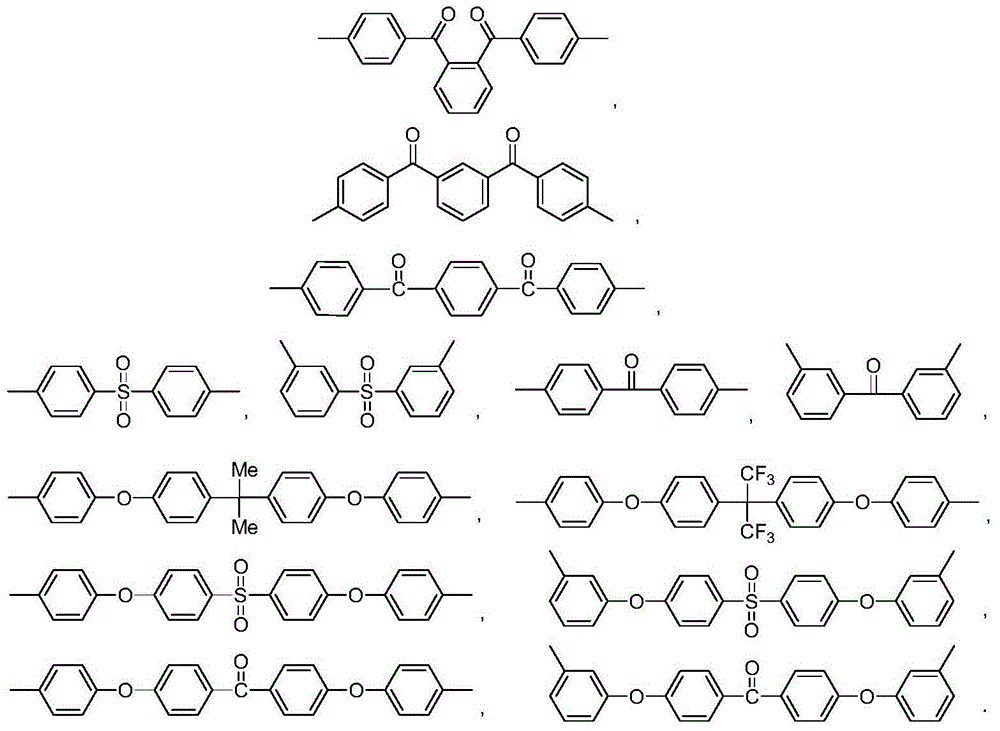
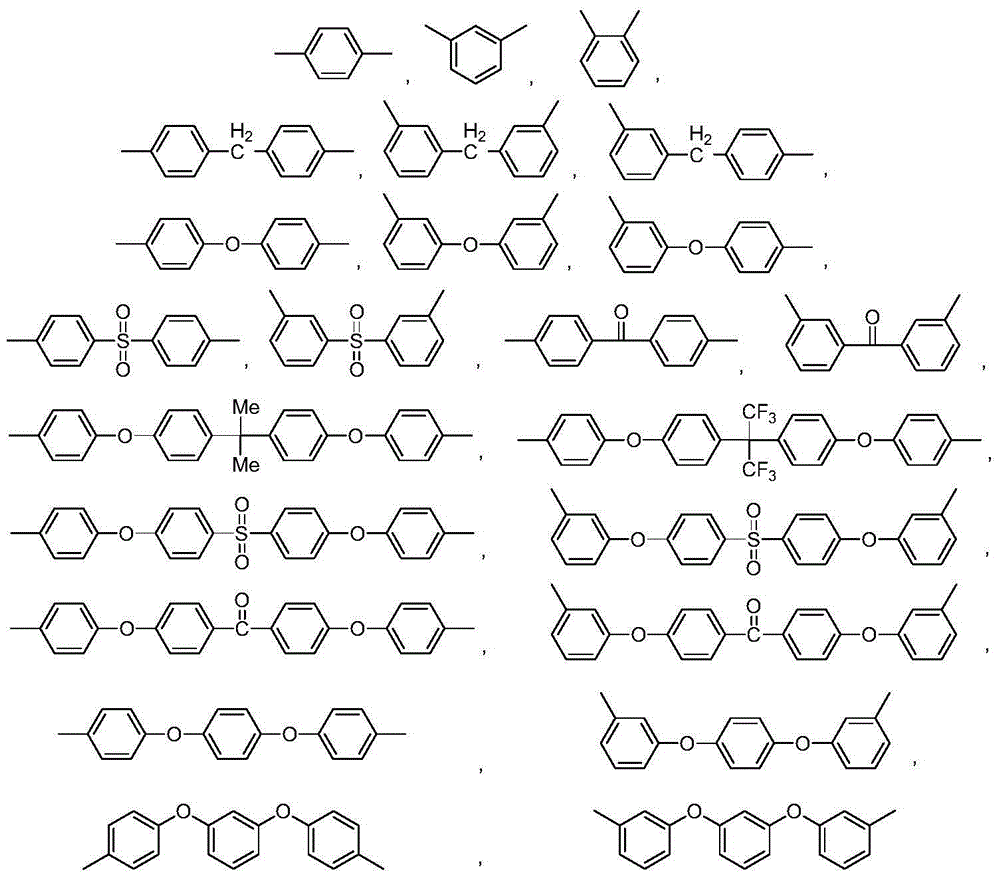
[0057] Add 4.58g (0.01mol) 4,4'-bisnitrophthalimide monomer, 2.18g (0.01mol) difluorobenzophenone, 6.36g (0.02mol) phenolphthalein into a dry and clean 500mL three-necked flask, 1.60g (0.04mol) sodium hydroxide, 25mL toluene, 50mL dimethylformamide, under the protection of nitrogen, reflux with water at 180°C for 10 hours; then, add the end-capping agent N-phenyl-4-nitro-o 0.0536 g (0.0002 mol) of phthalimide, continue to react for 2 hours; cool to room temperature, slowly pour the reaction solution into 500 mL of ethanol, filter, and boil and wash the obtained filter cake twice with ethanol, and then place it under vacuum at 120°C Dry in an oven for 8 hours to obtain 12.46 g of...
Embodiment 2
[0064] In this embodiment, the copolyimide has the following structural formula:
[0065]
[0066] Wherein, both m and n are integers greater than or equal to 1.
[0067] The reaction scheme of above-mentioned copolyimide is as follows:
[0068]
[0069] Its preparation method is as follows:
[0070] Add 5.29g (0.01mol) 4,4'-dichlorophthalimide monomer, 2.87g (0.01mol) dichlorodiphenyl sulfone, 6.36g (0.02mol) phenolphthalein into a dry and clean 500mL three-necked flask, 2.76g (0.02mol) potassium carbonate, 25mL toluene, 50mL sulfolane, under the protection of nitrogen, reflux with water at 200°C for 10 hours; then, add the end-capping agent N-phenyl-4-chlorophthaloyl Amine 0.0052g (0.0002mol), continue to react for 2 hours; cool to room temperature, slowly pour the reaction solution into 500mL ethanol, filter, the obtained filter cake is boiled and washed twice with ethanol, and then dried in a vacuum oven at 120°C for 8 hours , 7.88 g of light yellow copolyimide po...
Embodiment 3
[0077] In this embodiment, the copolyimide has the following structural formula:
[0078]
[0079] Wherein, both m and n are integers greater than or equal to 1.
[0080] The reaction scheme of above-mentioned copolyimide is as follows:
[0081]
[0082] Its preparation method is as follows:
[0083] Add 6.16g (0.01mol) 4,4'-dibromophthalimide monomer, 3.22g (0.01mol) phenolphthalein, 3.22g (0.01mol) monomer III difluorotri Benzophenone, 1.66g (0.012mol) of potassium carbonate, 25mL of toluene, 50mL of N-methylpyrrolidone, under the protection of nitrogen, reflux at 180°C with water for 10 hours; then, add the capping agent N-phenyl-4- Bromophthalimide 0.0604g (0.0002mol), continue to react for 2 hours; cool to room temperature, slowly pour the reaction solution into 500mL ethanol, filter, the obtained filter cake is boiled and washed twice with ethanol, and then placed in Dry in a vacuum oven at 120° C. for 8 hours to obtain 14.32 g of light yellow copolyimide powder...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thermal decomposition temperature | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com