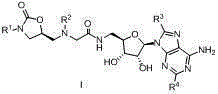
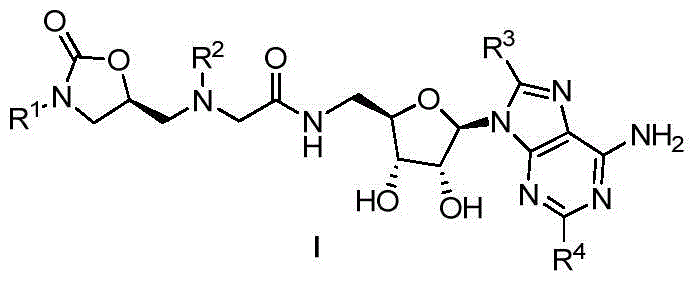
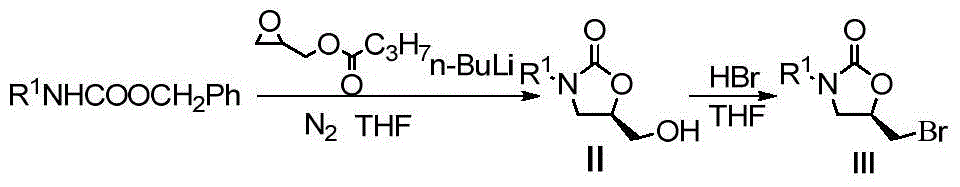Oxazolidinone-adenosine type multi-target antibacterial compound and its preparation method and application
A technology of oxazolidinone and oxazolidinone, which is applied in the application field of preparing antibacterial drugs, and achieves the effects of high antibacterial activity, good inhibition and killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: N-(((2R,3S,4R,5R)-5-(6-amino-9H-purin-9-yl)-3,4-dihydroxytetrahydrofuran-2-yl)methyl)- Preparation of 2-((((S)-3-(4-chlorophenyl)-2-oxooxazolidin-5-yl)methyl)(methyl)amine)acetamide (44)
[0025] Step 1: Add 3.90g (15mmol) benzyloxycarbonyl arylamine and 3.24g (22.5mmol) (R)-glycidyl butyrate to 24mLTHF, add 1.05g (16mmol) n-butyllithium after dissolution, and nitrogen protection The reaction was carried out for 5h, the reaction was completed, and concentrated to obtain 3.1g (R)-3-p-chlorophenyl-5-(hydroxymethyl)-2-oxazolone, and 3.1g (13mmol) (R)-3-p Chlorophenyl-5-(hydroxymethyl)-2-oxazolone was dissolved in 28mL THF. After dissolving, 2mL of 47% HBr aqueous solution was added and reacted at room temperature for 4h. After the reaction was completed, it was neutralized with saturated sodium bicarbonate solution. 210mL ethyl acetate extracted 3 times, the organic layer was washed with 30mL saturated brine, anhydrous MgSO 4 Dry, concentrate, and purify by colu...
Embodiment 2
[0041] Example 2: Extraction of TyrRS and determination of the activity of compounds on TyrRS
[0042] TyrRS from Staphylococcus aureus was expressed in E. coli and purified by Sephadex chromatography. The activity of TyrRS was determined by aminoacylation reaction. The enzyme reaction mixture consists of the following components: 100mM TrisHCl pH7.9, 50mMKCl, 16mMMgCl 2 , 5mMATP, 3mM dithiothreitol, 4mg / mL Escherichia coli MRE600tRNA and 10μM [3H]tyrosine (activity 1.48-2.22TBq / mmol). Mix and incubate TyrRS (0.2nM) and different concentrations of test substances at room temperature for 10 minutes, then add an equal amount of the above enzyme reaction mixture preheated to 37°C, and after co-incubating for 5 minutes, add an equal volume of 7% glacial trichloro Acetic acid solution was used to terminate the reaction, filtered through a 96-well Millipore filter plate, and the filtrate was detected by a scintillation counter, and each sample was repeated 4 times. The one withou...
Embodiment 3
[0043] Example 3: Determination of compound's transcriptional inhibitory activity on ribosome 50S subunit
[0044] The target compound was used to determine the inhibition of ribosomal 50S subunit transcription activity by a separate transcription method, and linezolid was used as a positive control. The purified S.aureus70S type ribosomes were suspended in TMK buffer (10mM Tris-HCl, pH7.4, 6mM MgCl, 60mMKCl, 1mM dithiothreitol), added different concentrations of test compounds, Promega amino acid mixture (make To a final concentration of 0.1 mM), 3 μL of PromegaS30 master mix, and (to a final concentration of) 200-800 nM of in vitro transcribed mRNA encoding firefly luciferase, the final volume of the transcription reaction was 10 μL. Read fluorescence value with Victor2V multifunctional microplate reader, IC 50 Calculated with MDLAssay Explorer software. Two independent experiments were carried out for each tested compound, and the average value was taken. The results are ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com