C-reactive protein quantitative determination kit and preparation method thereof
A reactive protein and quantitative determination technology, applied in the field of quantitative detection of C-reactive protein in blood, can solve the problems of unfavorable high-throughput automatic detection, low sensitivity, poor specificity, etc., and achieve an optimized chemiluminescence enhancement system, high sensitivity, small variation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
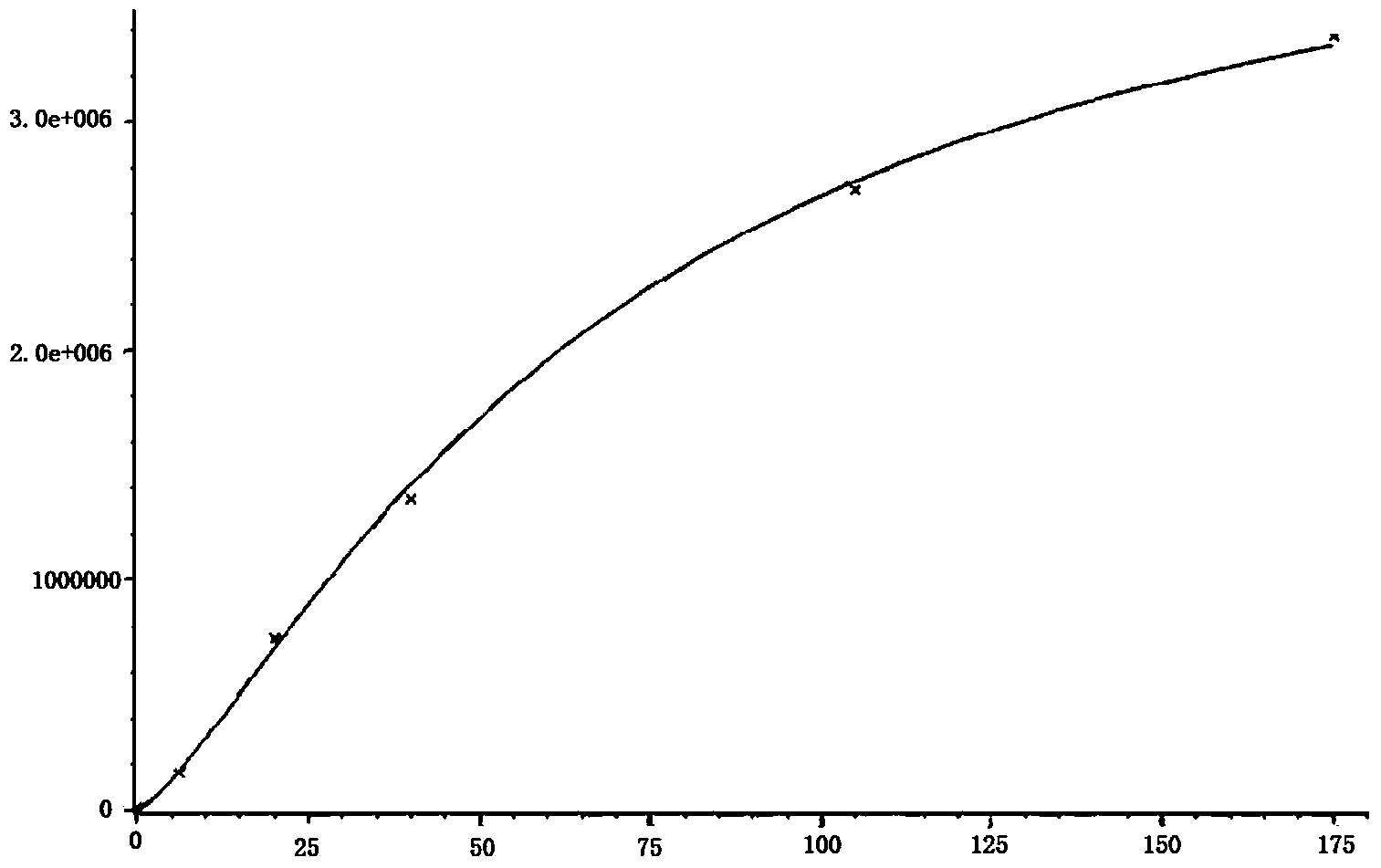
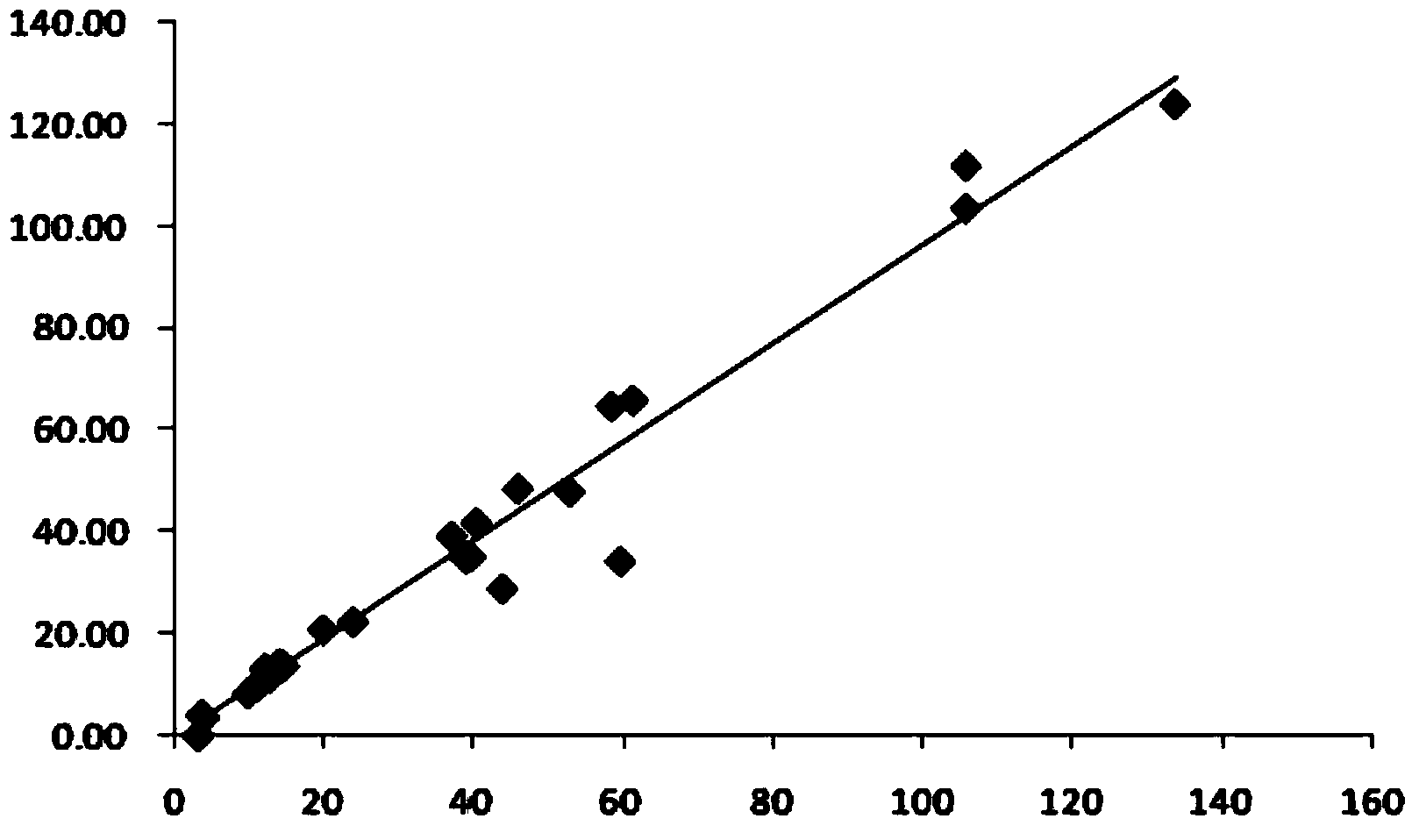
Image
Examples
Embodiment 1
[0048] Embodiment 1: the configuration of various buffers, specifically as follows:
[0049] 1. Tris salt buffer
[0050] Add 12.12g of Tris and 5.82g of sodium chloride into 1L of purified water, stir well until completely dissolved, and adjust the final pH to 7.5 with hydrochloric acid.
[0051] 2. Preparation of calibrator buffer
[0052] Add 0.01g of tetracycline and 0.1g of neomycin sulfate to 1L of newborn bovine serum, fully dissolve and process through 0.22μm filter membrane to prepare.
[0053] 3. Anti-reagent buffer
[0054] Add 30.5mg of Tris, 0.03g of tetracycline, 3g of sheep serum, 8g of newborn bovine serum, and 3g of horse serum into 1L of purified water, stir well until completely dissolved;
[0055] 4. Magnetic particle buffer
[0056] Add 12.12mg of Tris, 5.82mg of sodium chloride, and 50g of methyl cellulose ether into 1L of purified water, stir well until completely dissolved.
[0057] 5. Luminescence substrate buffer
[0058] Add 50.21g of Tris, 5.8...
Embodiment 2
[0061] Embodiment 2: Preparation of C-reactive protein quantitative assay kit
[0062] 1. Preparation of calibrators and quality controls
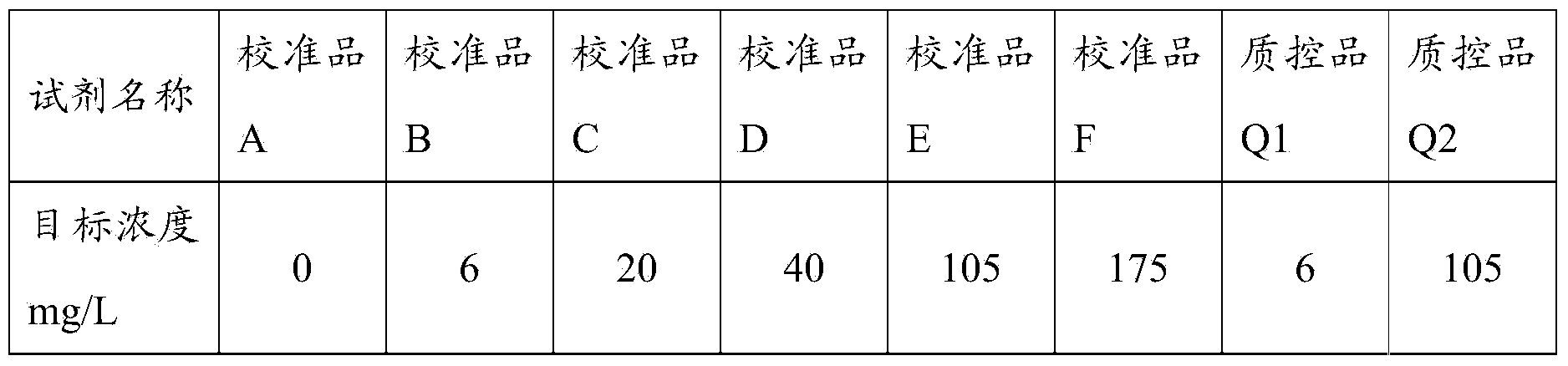
[0063] Firstly, the C-reactive protein was dissolved in the standard buffer solution, and prepared as a calibrator and a quality control product with target concentrations as shown in Table 1; the C-reactive protein used in this example was purchased from the manufacturer Fitzgerald Company.
[0064] Table 1 Preparation of calibrators and quality controls
[0065]
[0066] 2. The preparation method of the anti-reagent is as follows:
[0067] 1) Coupling of fluorescein isothiocyanate and C-reactive protein antibody to obtain fluorescein isothiocyanate-labeled C-reactive protein-coated antibody:
[0068] First, use anti-reagent buffer to prepare FITC solution with a concentration of 2.5mg / mL, and then make C-reactive protein:FITC solution=1:1.1 according to the mass ratio Transfer the two to a brown glass bottle and stir at room temper...
Embodiment 3
[0079] Embodiment 3: The method for detecting C-reactive protein using the C-reactive protein quantitative assay kit described in Example 2, the method comprises the steps of:
[0080] 1) Take three test tubes and add 15 μL C-reactive protein calibrator, 15 μL LC-reactive protein quality control, and 15 μL sample to be tested;
[0081] 2) Add 60 μL of anti-reagent to each test tube, cover the test tube with a plastic film, shake the test tube gently for 30 seconds, and place it in a water bath at 37°C for 5 minutes;
[0082] 3) Add 30 μL of magnetic particle reagent to each test tube, cover the test tube with a plastic film, shake the test tube gently for 30 seconds, and place it in a water bath at 37°C for 5 minutes;
[0083] 4) Precipitate the test tube on the magnetic separator for 2 minutes, slowly invert the test tube and the magnetic separator, pour out the supernatant, put the inverted test tube together with the magnetic separator on the filter paper, and pat the botto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com