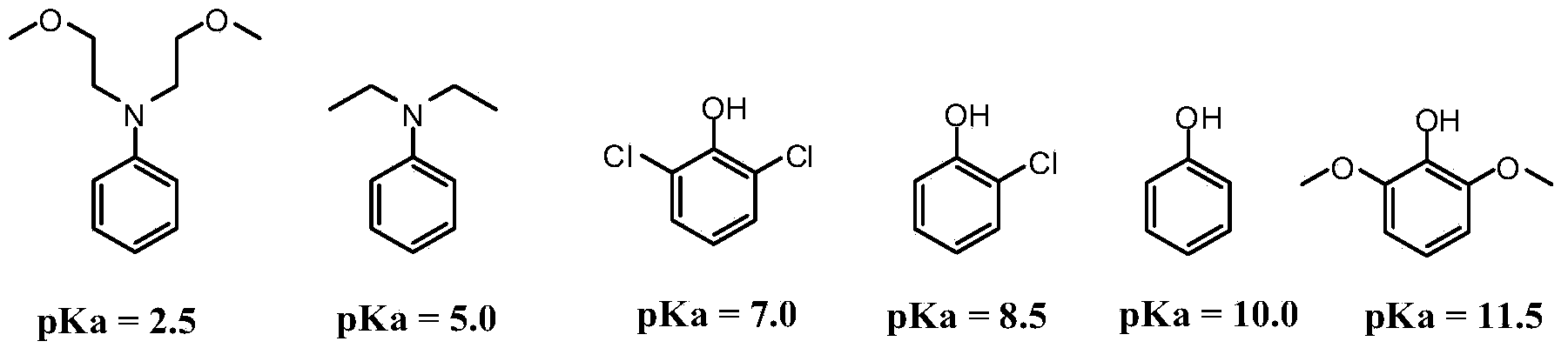
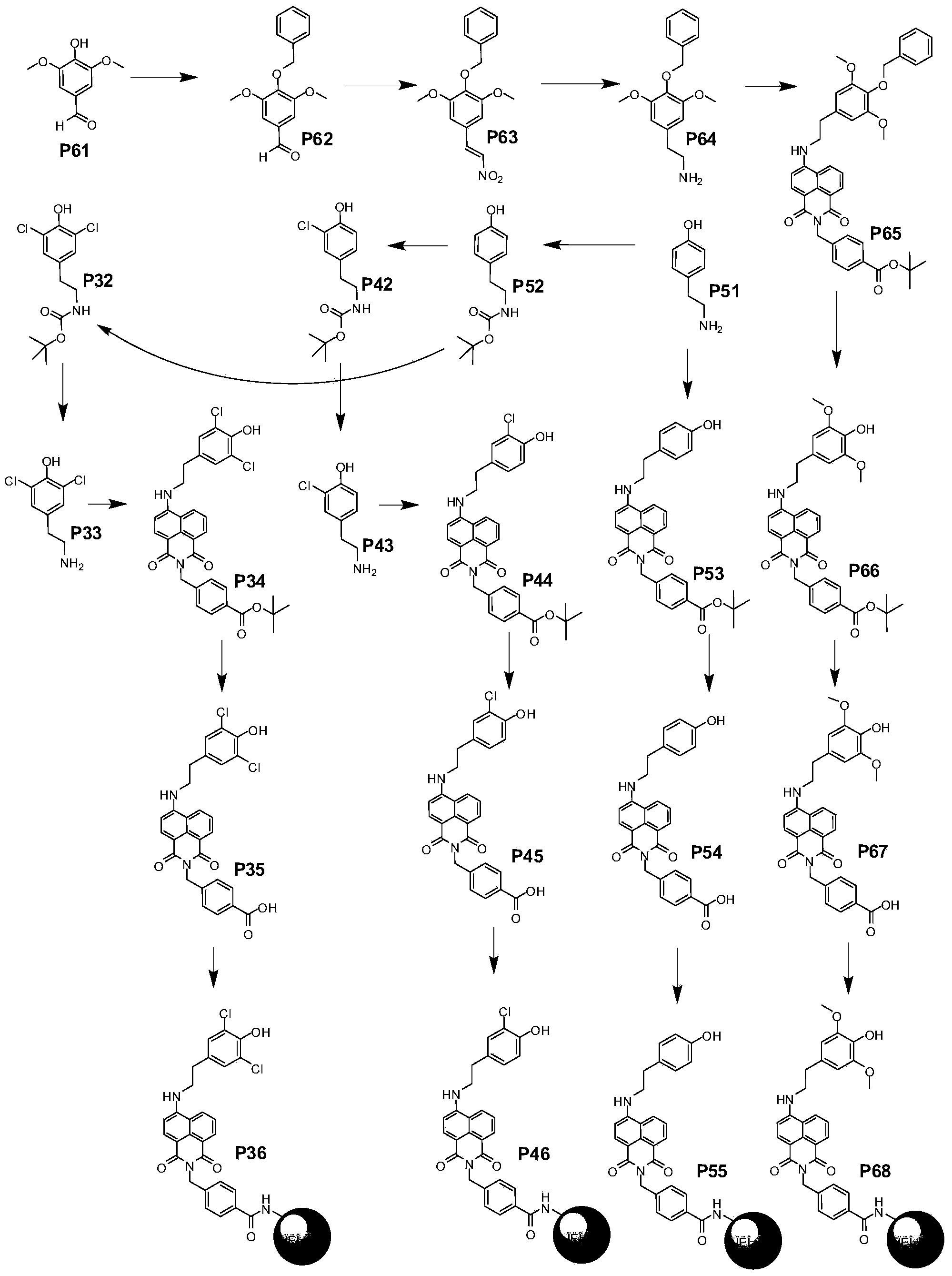Organic compound for preparing wide pH fluorescence probes and application thereof
A technology of organic compounds and fluorescent probes, which can be used in organic chemistry, organic dyes, pH testing, etc., and can solve the problems of easy damage, high impedance, and inability to measure a wide range of pH values.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
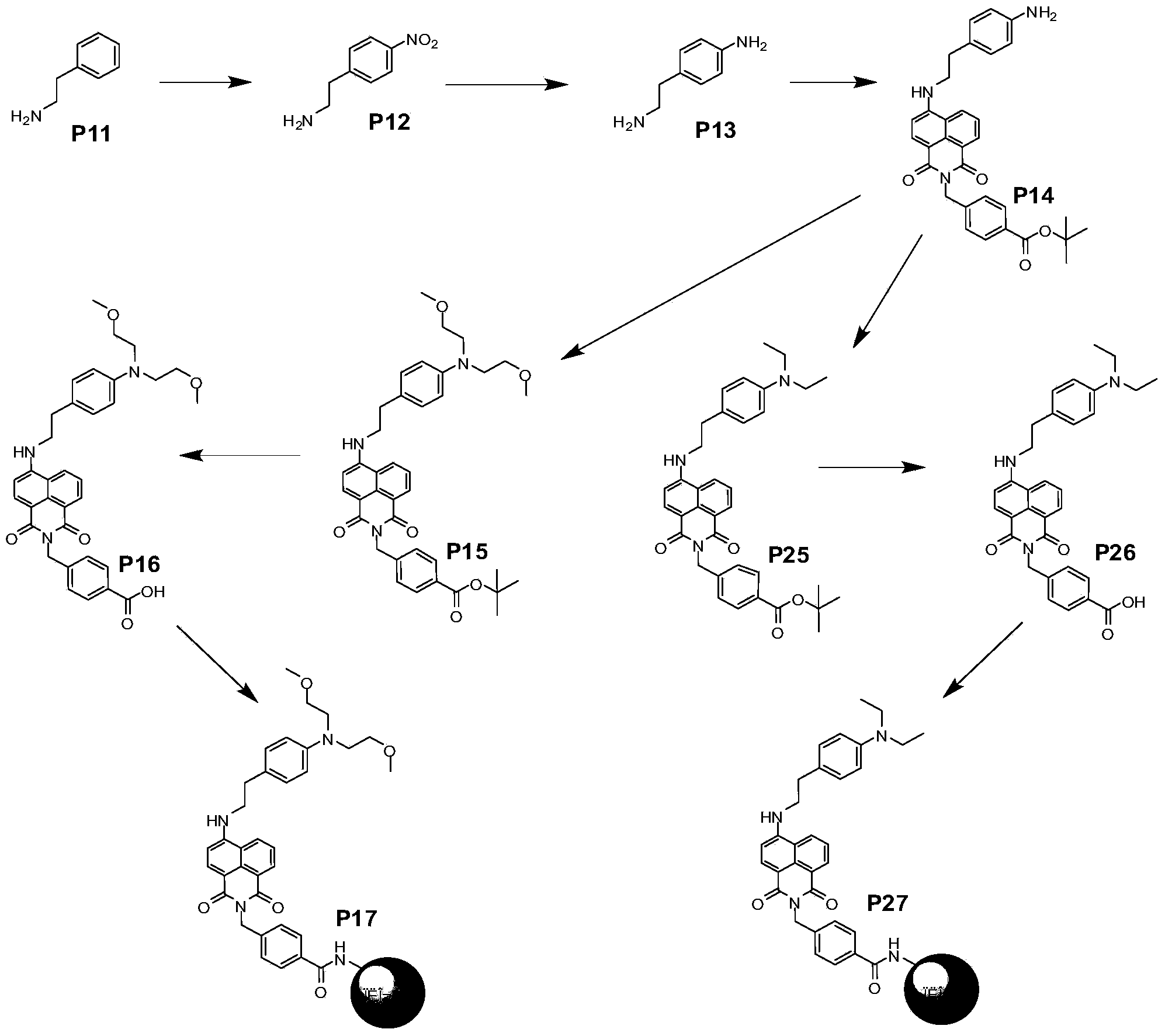
Embodiment 1
[0082] Synthesis of Compound P12:
[0083] Phenylethylamine (P11) (60.5 g 0.50 mol) was mixed with concentrated hydrochloric acid (50 ml, 0.6 mol). The formed salt was dissolved in 65 ml of water. Slowly add this solution dropwise to 600ml of 35% nitric acid solution under rapid stirring at room temperature. Stirring was continued overnight after the dropwise addition was complete. The resulting yellow precipitate was filtered, dissolved in 300ml of water, and the pH was greater than 12 with 40% sodium hydroxide, extracted with dichloromethane (3x300ml), the resulting solution was dried with anhydrous potassium carbonate, filtered, and spin-dried to obtain 61.5g of the product (74%). 1H NMR (CDCl 3 ) δ = 2.80(t, 2H), 2.64(s, br, 2H), 2.94(t, 2H), 7.55(d, 2H), 8.25(d, 2H).
[0084] Synthesis of Compound P13:
[0085] Dissolve nitrophenethylamine (P12) (61.5g 0.37mol) in ethanol (400ml), add 3.3g of 5% palladium carbon, and carry out catalytic hydrogenation under normal pr...
Embodiment 2
[0093] Synthesis of Compound P25
[0094] Add compound P14 (2.0g, 3.83mmol), ethyl iodide (2.43g, 15.32mmol), potassium iodide (2.54g, 15.32mmol), DIEA 2.3mL (15.30mmol) and 12ml NMP in a 50ml single-necked bottle, 110°C Stir for 24 hours. After the reaction is complete, pour into 280ml of ice water. The resulting yellow precipitate was filtered and washed three times with 40 ml of water. The resulting crude product was purified by a silica gel column to obtain 1.83 g (83%) of a yellow solid. 1H NMR (300MHz, CDCl3): 1.55(s, 9H), 1.25(t, 6H), 3.53(q, 4H), 2.97(t, 2H), 3.35(t, 2H), 5.38(s, 2H), 6.68 (t, 2H), 7.56-7.55 (m, 3H) 7.85 (d, 2H) 8.05 (d, 2H) 8.40 (d, 2H) 8.60 (d, 2H).
[0095] Synthesis of compound P26: Weigh compound P25 (0.16g, 0.28mmol) into 4ml of dichloromethane, then add 2ml of trifluoroacetic acid, stir at room temperature for 30min, spin off the solvent to obtain 0.18g of a yellow solid, and proceed directly to the next step.
[0096] The synthesis of compo...
Embodiment 3
[0098] Synthesis of compound P52: slowly dissolve tyramine (10 g, 72.90 mmol) in THF (ie tetrahydrofuran, 140 ml) and methanol (20 ml), then add triethylamine (7.36 g, 72.90 mmol). The solution was cooled to 0°C with an ice-salt bath, and di-tert-butyl carbonate (8.20 g, 87.48 mmol) was added in batches. After the addition was complete, it was stirred at 0°C for 1 hour, then at room temperature overnight. The solvent was spin-dried to obtain 18.0 g (91%) of the product. 1 H NMR (300MHz, DMSO-d6): δ9.12(s, 1H), 6.96(d, J=8.1Hz, 2H), 6.78(t, J=5.1Hz, 1H), 6.66(d, J=8.4 Hz, 2H), 3.00-3.07(m, 2H), 2.54(t, J=7.8Hz, 2H), 1.35(s, 9H). 13 C NMR (DMSO-d6): δ156.256, 130.125, 130.102, 115.737, 78.108, 42.602, 35.426.28.934.
[0099] Synthesis of compound P32: Compound P52 (8.0 g, 33.71 mmol) was dissolved in 160 ml of anhydrous dichloromethane, and heated to reflux under nitrogen protection. Dissolve 13.65 g (101.41 mmol) of sulfonyl chloride in 100 ml of anhydrous dichloromethane a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ionic strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com