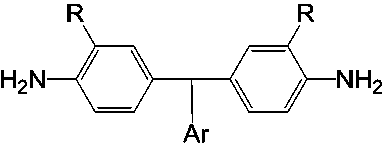
Diamine monomers containing asymmetric non-coplanar structure and preparation method thereof
A non-coplanar, diamine monomer technology, applied in the field of aromatic diamine monomer and its preparation, can solve problems such as reducing the thermal stability of polyimide, and achieve low cost, simple operation, high yield and high purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
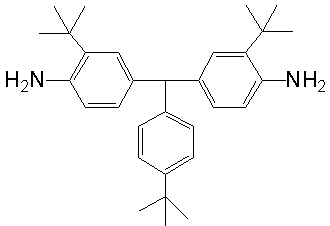
[0031] Under nitrogen protection, mix 15 grams (0.100 moles) of 2-tert-butylaniline and 6.5 grams (0.040 moles) of 4-tert-butylbenzaldehyde, and add 3.6 grams (0.024 moles) of the catalyst dropwise within 1 hour at room temperature Trifluoromethanesulfonic acid, be warming up to 160 DEG C and reflux for 20 hours after finishing dropping, cool to room temperature after finishing the reaction, then gradually add dropwise the ammonia solution that the mass percent concentration is 26% to be neutralized to neutrality, will precipitate out and filter, Dry overnight, and the obtained crude product is further purified by silica gel column chromatography (the eluent is a mixture of ethyl acetate and petroleum ether with a volume ratio of 1:8), and finally a light yellow powdery solid is obtained. Non-coplanar diamine monomer (3,3'-di-tert-butyl-4,4'-diaminophenyl-4''-tert-butyltoluene).
[0032] The productive rate in the present embodiment is about 90%; Melting point is 87.5 ℃ (measu...
Embodiment 2
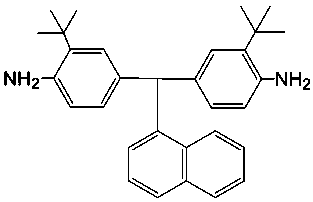
[0034] Under nitrogen protection, mix 15 grams (0.100 moles) of 2-tert-butylaniline and 6.0 grams (0.038 moles) of 4-tert-butylbenzaldehyde, and add 3.6 grams (0.024 moles) of the catalyst dropwise within 1 hour at room temperature Trifluoromethanesulfonic acid, be warming up to 160 DEG C and reflux for 20 hours after the dropwise addition, cool to room temperature after the completion of the reaction, then gradually add dropwise an ammonia solution with a mass percentage concentration of 27% to neutralize, and filter the precipitate. Dry overnight, and the obtained crude product is further purified by silica gel column chromatography (the eluent is a mixture of ethyl acetate and petroleum ether with a volume ratio of 1:6), and finally a white powdery solid is obtained that contains asymmetric asymmetry. Coplanar diamine monomer (3,3′-di-tert-butyl-4,4′-diaminophenyl-1-naphthalene methane).
[0035] The productive rate in the present embodiment is about 88%; Melting point is 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com