Fluorescence chemical sensor for detecting 2,4,6-trinitrophenol and preparation method of fluorescence chemical reactor
A chemical sensor, trinitrophenol technology, applied in chemical instruments and methods, fluorescence/phosphorescence, luminescent materials, etc., can solve the problems of huge instrument and equipment operation process, inability to identify and detect, difficult to quickly detect, etc., to achieve excellent Effects of thermal and chemical stability, high sensitivity, and good fluorescence characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
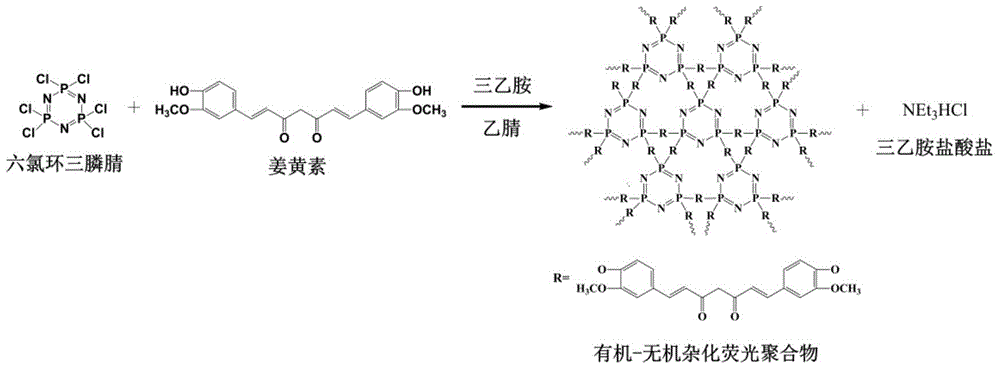
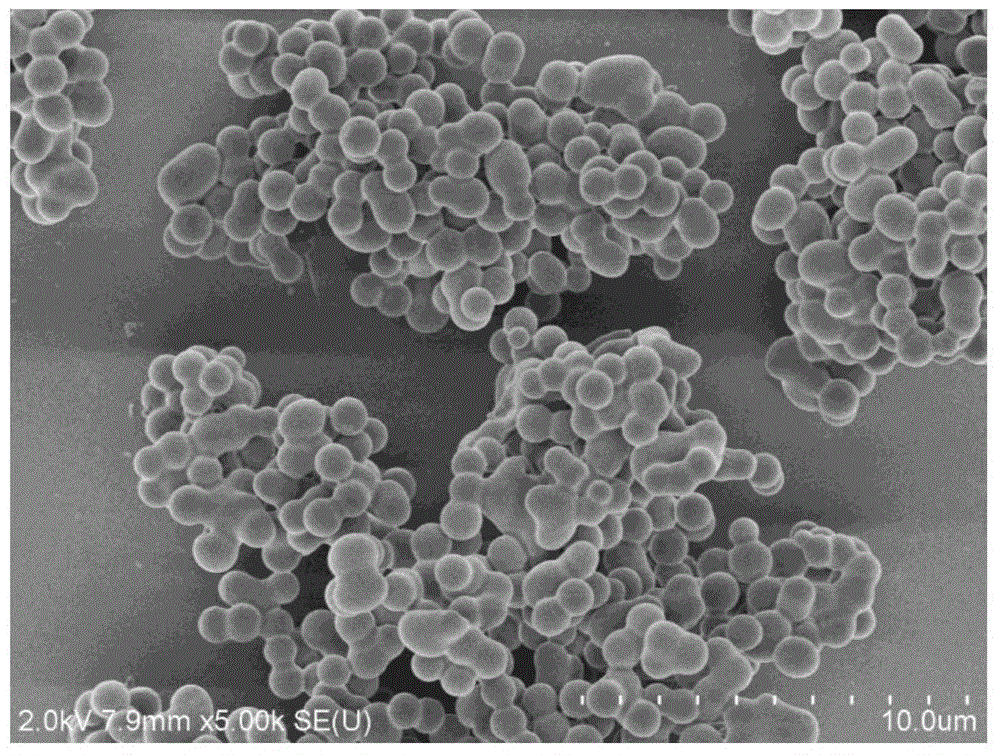
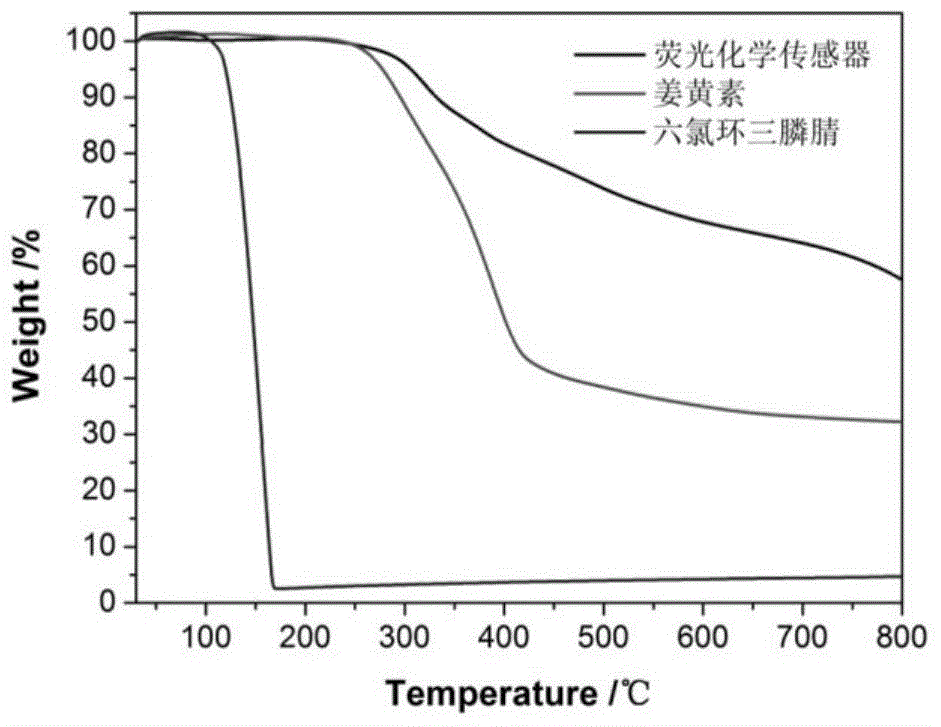
[0021] In a 250mL dry single-necked round bottom flask, 0.100g of hexachlorocyclotriphosphazene and 0.320g of curcumin (the molar ratio of hexachlorocyclotriphosphazene and curcumin is 1:3) were dissolved in 50mL of acetonitrile, and then added to the above 2 mL of triethylamine was added to the solution, and the reaction was carried out at room temperature in an ultrasonic water bath (100 W) for 7 hours. After the reaction, the solid product was separated by centrifugation, washed several times with acetone and deionized water until the supernatant was colorless after centrifugation, and finally dried in vacuum at 65°C for 24 hours to obtain a yellow solid powder, which was the fluorescent chemical sensor.
[0022] Implementation effect of this embodiment: figure 1 is a schematic diagram of the preparation route of the fluorescent chemical sensor. Hexachlorocyclotriphosphazene and curcumin undergo a one-step polycondensation reaction under the condition of triethylamine as a...
Embodiment 2
[0027] In a 250mL dry single-necked round bottom flask, 0.400g hexachlorocyclotriphosphazene and 1.280g curcumin (the molar ratio of hexachlorocyclotriphosphazene and curcumin is 1:3) were dissolved in 100mL acetonitrile, and then added to the above Add 4 mL of triethylamine to the solution, and react in an ultrasonic water bath (250W) at room temperature for 5 hours. After the reaction, the solid product was separated by centrifugation, washed several times with acetone and deionized water until the supernatant was colorless after centrifugation, and finally dried in vacuum at 65°C for 24 hours to obtain a yellow solid powder, which was the fluorescent chemical sensor.
Embodiment 3
[0029] In a 250mL dry single-necked round bottom flask, 0.100g of hexachlorocyclotriphosphazene and 0.320g of curcumin (the molar ratio of hexachlorocyclotriphosphazene and curcumin is 1:3) were dissolved in 50mL of acetonitrile, and then added to the above Add 2 mL of triethylamine to the solution, and react in an ultrasonic water bath (300W) at 60°C for 5 hours. After the reaction, the solid product was separated by centrifugation, washed several times with acetone and deionized water until the supernatant was colorless after centrifugation, and finally dried in vacuum at 65°C for 24 hours to obtain a yellow solid powder, which was the fluorescent chemical sensor.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com