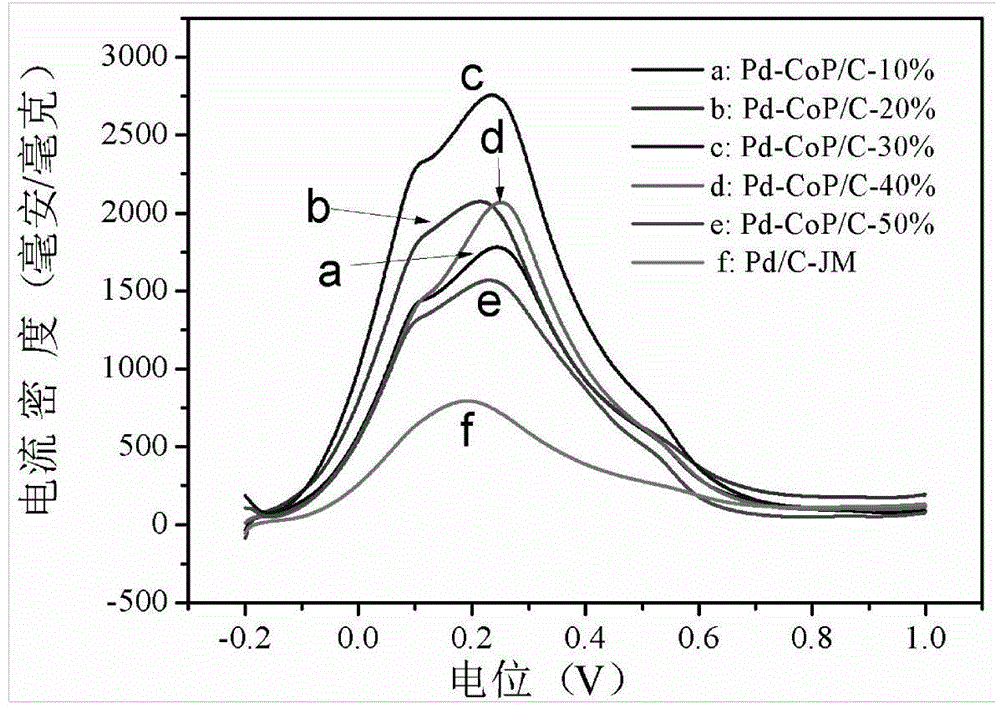
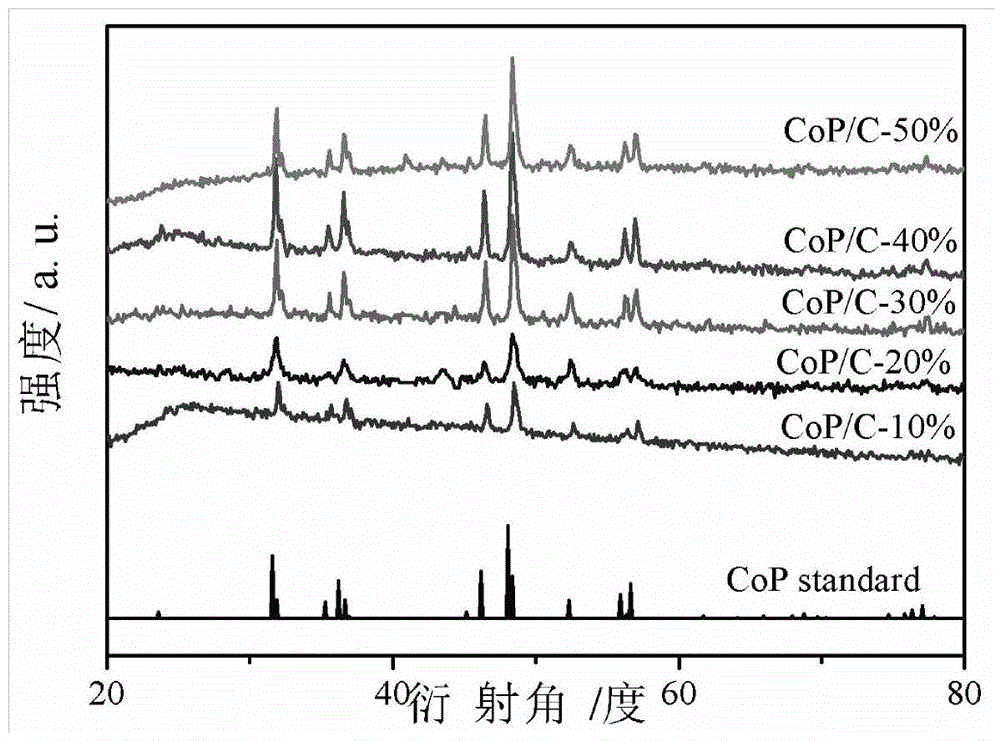
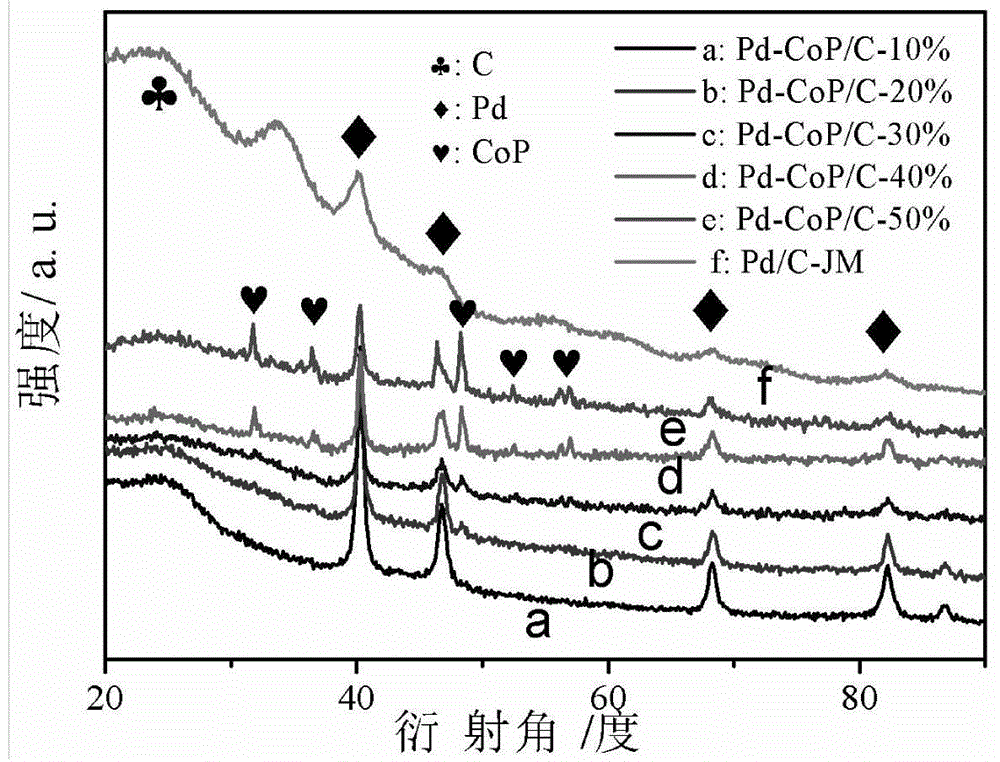
Preparation method of high-performance superlow-palladium-capacity anode electrocatalyst Pd-CoP/C of direct formic acid fuel cell
A technology of fuel cells and electrocatalysts, which is applied to battery electrodes, circuits, electrical components, etc., can solve problems limited to the research stage, reduce the utilization rate of precious metals, and limited catalytic activity, and achieve simple processing methods, excellent electrochemical performance, Simple operation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Add 0.60g of cobalt chloride hexahydrate and 1.6889g of Vulcan XC-72 produced by American Cabot Company into a beaker containing 50mL of secondary water, ultrasonicate for 30min, stir for 60min, then put it in a muffle furnace and evaporate to dryness at 100°C Moisture content to obtain a black mixture, then 2.016g of the black mixture and 1.32g of sodium hypophosphite hydrate were added to a quartz boat, placed in a tube furnace, and calcined at 800°C for 60min in a nitrogen atmosphere, wherein the oxygen flow rate was 80cc min -1 , tube furnace from room temperature to 5 ℃ min -1 The heating rate was increased to 800° C., and the prepared composite carrier was marked as CoP / C-10% (wherein 10% represents the mass percentage of CoP in the composite carrier).
[0049] (2) Weigh 190 mg of CoP / C-10% prepared in step (1), add it into a beaker containing 100 mL of ethylene glycol, and add chloropalladium acid containing 10 mg of palladium at the same time, stir for 5 h a...
Embodiment 2
[0065] (1) Add 1.20g of cobalt chloride hexahydrate and 1.5016g of Vulcan XC-72 produced by American Cabot Company into a beaker containing 50mL of secondary water, ultrasonicate for 30min, stir for 60min, then put it in a muffle furnace and evaporate to dryness at 110°C The black mixture was obtained by the water content, and then 2.157g of the black mixture and 2.64g of sodium hypophosphite hydrate were added to a quartz boat, placed in a tube furnace, and calcined at 800°C for 90min in a nitrogen atmosphere, wherein the oxygen flow rate was 80cc min -1 , tube furnace from room temperature to 5 ℃ min -1 The heating rate was increased to 800° C., and the prepared composite carrier was marked as CoP / C-20% (where 20% represents the mass percentage of CoP in the composite carrier).
[0066] (2) Weigh 190 mg of CoP / C-20% prepared in step (1), add it into a beaker containing 100 mL of ethylene glycol, and add chloropalladium acid containing 10 mg of palladium at the same time, sti...
Embodiment 3
[0072] (1) Add 1.20g of cobalt chloride hexahydrate and 0.876g of Vulcan XC-72 produced by American Cabot Company into a beaker containing 50mL of secondary water, ultrasonicate for 30min and stir for 60min, then put it into a muffle furnace and evaporate to dryness at 120°C The water content gives a black mixture, and then 1.5313g of the black mixture and 2.64g of sodium hypophosphite hydrate are added to a quartz boat, placed in a tube furnace, and calcined at 800°C for 120min in a nitrogen atmosphere, wherein the oxygen flow rate is 80cc min -1 , tube furnace from room temperature to 5 ℃ min -1 The heating rate was increased to 800° C., and the prepared composite carrier was marked as CoP / C-30% (wherein 30% represents the mass percentage of CoP in the composite carrier).
[0073] (2) Weigh 190 mg of CoP / C-30% prepared in step (1), add it into a beaker containing 100 mL of ethylene glycol, and add chloropalladium acid containing 10 mg of palladium at the same time, stir for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum power density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com