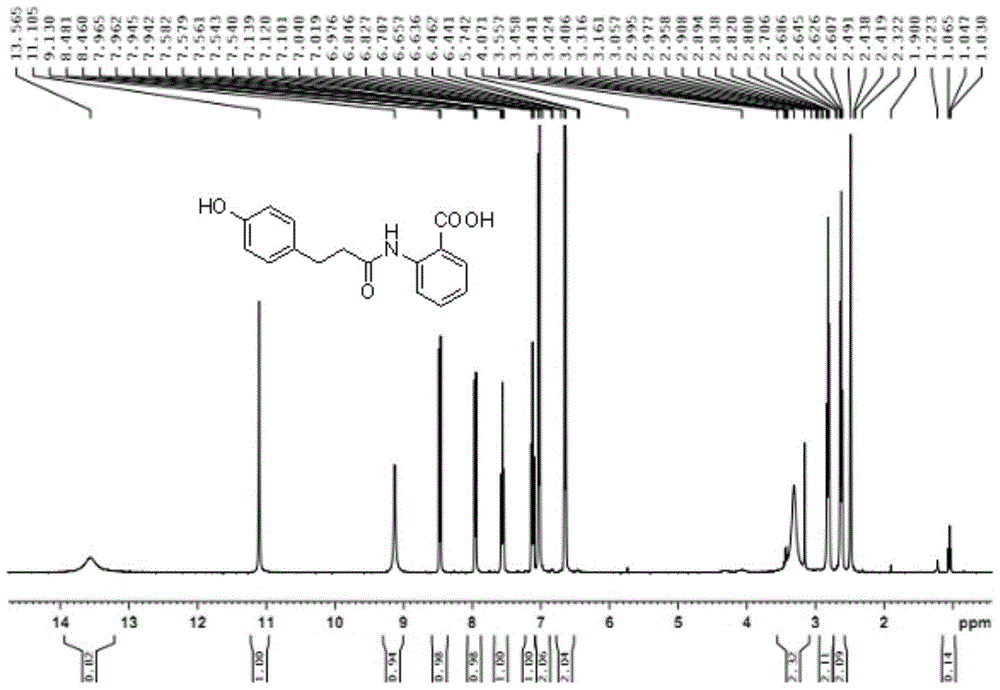
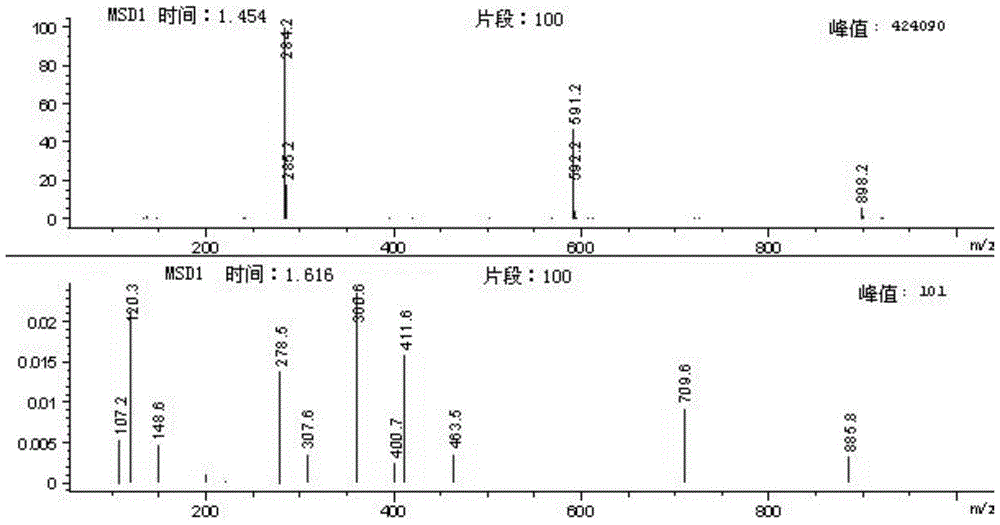
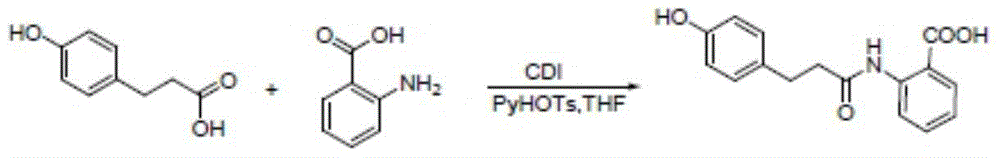A kind of dihydroovenalyl anthranilic acid D synthetic method
A technology of avenyl o-aminobenzoic acid, which is applied in chemical instruments and methods, preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve the problems of emission and high production cost, and achieve emission avoidance, high controllability, and lightening pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
[0033] Add p-hydroxyphenylpropionic acid (3 g, 0.018 mol), DMF (0.25 ml, 0.0036 mol) and dichloromethane (100 ml) respectively into a single-necked bottle, and stir at room temperature at 18°C. Thionyl chloride (2ml, 0.027mol) was added dropwise to the suspension, stirred at room temperature for about 3 hours, the system gradually changed from slightly cloudy to a clear yellow solution, and methyl anthranilate (2.7g, 0.018mol) was continued to be added dropwise. ), after dropping, a large amount of white solids were precipitated, then sodium carbonate (3g, 0.0283mol) was added, and the system became very viscous. After 3 hours of reaction, TLC spotting (take a little sample, add water and ethyl acetate, organic Layer spot plate, developer ethyl acetate:petroleum ether=1:5 (V / V)), generate a principal point. After 4 hours, the board was tapped, the raw materials decreased, but the solution was acidic, and 1 gram of sodium carbonate was added to continue the reaction...
Embodiment 2
[0036] Add p-hydroxyphenylpropionic acid (4.58 g, 0.03 mol), DMF (0.4 ml, 0.003 mol) and dichloromethane (50 ml) respectively into a single-necked bottle, stir at room temperature or in a cold water bath (10°C). Thionyl chloride (3.6ml, 0.05mol) was added dropwise to the suspension, and stirred at room temperature for about 12 hours. The system gradually changed from a slightly turbid to a clear yellow solution, and methyl anthranilate (4.53g, 0.03mol) was added dropwise. ), after dropping, a large amount of white solids were precipitated, and sodium hydroxide (5.28g, 0.132mol) was added in batches. After 12 hours of reaction, the pH value was weakly alkaline, and dichloromethane was recovered by rotary evaporation to obtain solids, and water was added Add 30ml of methanol and 30ml of sodium hydroxide solution (2.14g, 0.066mol) with a concentration of 10mol / l, keep the pH value of the liquid in the one-necked bottle at 12, stir at room temperature for about 2 hours, the system ...
Embodiment 3
[0038]Add p-hydroxyphenylpropionic acid (3.0 g, 0.018 mol), DMF (0.25 ml, 0.0036 mol) and dichloromethane (50 ml) respectively into a single-necked bottle, and stir at 18°C. Add thionyl chloride (2ml, 0.027mol) dropwise to the suspension, stir at room temperature for about 0.5 hours, the system gradually changes from slightly turbid to a clear yellow solution, add methyl anthranilate (2.7g, 0.018mol) dropwise After dropping, a large amount of white solids precipitated, and disodium hydrogen phosphate trihydrate (4.93g, 0.0823mol) was added in batches, and after 12 hours of reaction, dichloromethane was recovered by rotary evaporation to obtain a solid, and 30 ml of water and ethanol were added , and add sodium hydroxide solution (2.14g, 0.066mol) with a concentration of 10mol / l, keep the pH value of the liquid in the one-mouth bottle at 12, stir at room temperature for about 24 hours, the system is milky white, add concentrated hydrochloric acid to adjust the pH value to 6 , a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com