Preparation method for intermediate (5E, 9E)-farnesyl acetone of teprenone
A technology of acacia acetone and teprenone, which is applied in the field of chemical synthesis of chemicals and intermediates, can solve the problems of low product purity, difficulty in large-scale production and the like, and achieves high purity, easy pollution treatment, and yield. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
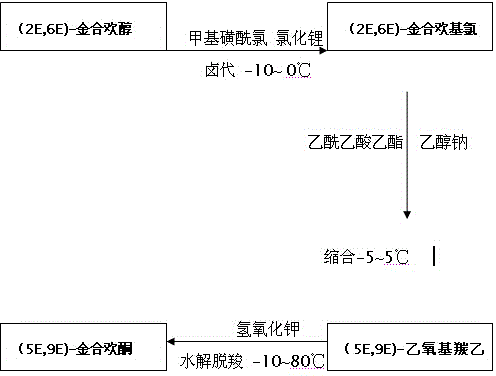
[0042] 1. Synthesis of (2E,6E)-farnesyl chloride
[0043]
[0044] Add 21.51g of triethylamine to 43.14g of (2E,6E)-farnesol, then add 8.23g of lithium chloride dissolved in 100ml of DMF, cool down to 0°C, add dropwise 16.56ml of methanesulfonyl chloride, 10 The dropwise addition was completed in 1 minute, and the temperature was controlled at 0°C for 2 hours. No raw materials remained. Wash with 5% aqueous sodium bicarbonate solution and 5% normal saline, dehydrate with anhydrous magnesium sulfate, and desolventize the filtrate under reduced pressure to obtain 44.81 g of light yellow oily liquid.
[0045] 2. Synthesis of (5E,9E)-Ethoxycarboethyl Albizia Acetone
[0046]
[0047] Add 24.5g of sodium ethylate to 98ml of absolute ethanol, cool down to 0°C, add 46.85g of ethyl acetoacetate dropwise, maintain the temperature for 20 minutes, add dropwise 55ml of 43.29g of (2E, 6E )-Farnesyl chloride in dioxane solution, after the dropwise addition, naturally rise to r...
Embodiment 2
[0052] 1. Synthesis of (2E,6E)-farnesyl chloride
[0053]
[0054] Add 21.51g of triethylamine to 43.14g of (2E,6E)-farnesol, then add 8.23g of lithium chloride dissolved in 100ml of DMF, cool down to -10°C, add dropwise 16.56ml of methanesulfonyl chloride, The dropwise addition was completed in 10 minutes, and the temperature was controlled at 0°C for 2 hours. No raw materials remained. Pour the reaction liquid into 500ml ice water, extract with 3×100ml n-hexane, combine the organic phases, and use 5% potassium bisulfate aqueous solution and purified water respectively. , 5% aqueous sodium bicarbonate solution, 5% normal saline washing, dehydration with anhydrous magnesium sulfate, and desolvation of the filtrate under reduced pressure to obtain 44.01 g of light yellow oily liquid.
[0055] 2. Synthesis of (5E,9E)-Ethoxycarboethyl Albizia Acetone
[0056]
[0057] Add 24.5g of sodium ethylate to 98ml of absolute ethanol, cool down to 0°C, add 46.85g of ethyl ac...
Embodiment 3
[0062] 1. Synthesis of (2E,6E)-farnesyl chloride
[0063]
[0064] Add 21.51g of triethylamine to 43.14g of (2E,6E)-farnesol, then add 8.23g of lithium chloride dissolved in 100ml of DMF, cool down to 0°C, add dropwise 16.56ml of methanesulfonyl chloride, 10 The dropwise addition was completed in 1 minute, and the temperature was controlled at 0°C for 2 hours. No raw materials remained. Wash with 5% aqueous sodium bicarbonate solution and 5% normal saline, dehydrate with anhydrous magnesium sulfate, and desolventize the filtrate under reduced pressure to obtain 44.55 g of light yellow oily liquid.
[0065] 2. Synthesis of (5E,9E)-Ethoxycarboethyl Albizia Acetone
[0066]
[0067] Add 24.5g of sodium ethylate to 98ml of absolute ethanol, cool down to 0°C, add 46.85g of ethyl acetoacetate dropwise, maintain the temperature for 20 minutes, add dropwise 55ml of 43.29g of (2E, 6E )-Farnesyl chloride in dioxane solution, after the dropwise addition, naturally rise to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com