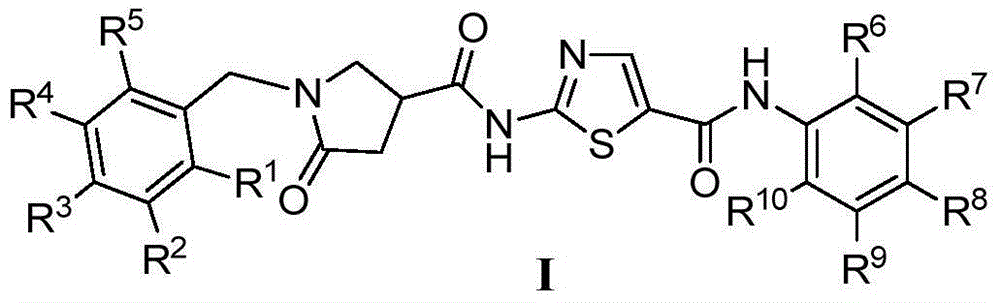
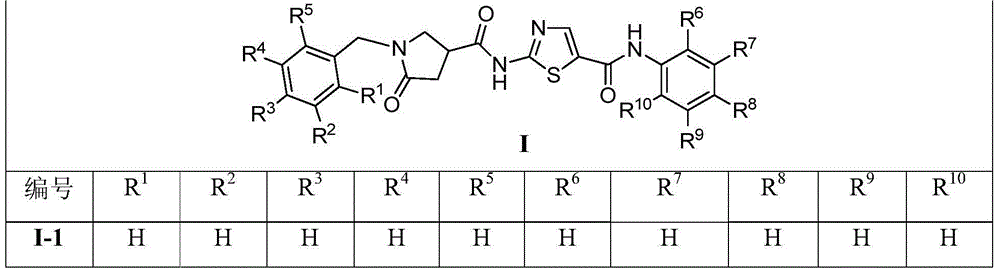
1-benzyl-2-pyrroline ketone-4-amide compounds and preparing method and application thereof
A technology of amide compounds and pyrrolidone, which is applied in the direction of drug combination, organic chemistry, and pharmaceutical formulations, to achieve the effect of inhibiting cell proliferation and angiogenesis and significant curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]
[0055] A1-Benzyl-2-pyrrolidinone-4-carboxylic acid
[0056]
[0057] With itaconic acid (18g, 0.138mol) and benzylamine (14.8g, 0.138mol) in reaction bottle, N 2 Under protection, heat up to 130°C, slowly melt and stir, react for 2.5h, stop heating, when cooled to 100°C, add 200ml of 10% NaOH solution under stirring, cool to room temperature, wash the aqueous layer with ethyl acetate, wash with 10 % hydrochloric acid solution was added dropwise to the water layer, a large amount of white solids were formed until pH = 1-2, filtered and washed with water until the pH was about 6 to obtain 25.1 g of white granular solids, yield 82.9%, mp143-145 ° C, HRMS =220.0886[M+H] + .
[0058] B1-Benzyl-2-pyrrolidinone-4-formyl chloride
[0059]
[0060] Add 30ml of dichloromethane to the above compound 1A (6g, 27.4mmol), heat to boiling, gradually dissolve, slowly add 3.2ml (44.0mmol) of thionyl chloride dropwise under stirring, continue to react for 24h, cool naturally...
Embodiment 2
[0065] Example 2: In vitro VEGFR2 inhibitory activity test.
[0066] VEGFR2 enzyme system test solution: 50mM Tris-HCl pH 7.5 buffer, 5mM MnCl 2 , 5 mM MgCl 2 , 0.01% Tween-20, and 2 mM DTT, containing 10 μM ATP, 0.1 μg / mL biocylated polyglutamic acid / tyrosine (4:1), and 0.1 nM VEGFR2 (Millipore, UK).
[0067] ATP catalytic inhibition: Compounds and enzymes were incubated at room temperature for 5min, then added 25μL 100mM EDTA solution, 10μg / mL AlphaScreen streptavidin protein donor and 10μg / mL acceptor 62.5mM HEPES pH7.4 solution and 250mM A solution of NaCl and 0.1% BSA. Detection was carried out by a microplate reader, and the concentration of the test substance when the inhibition rate was 50% was calculated.
[0068] Among them, 15 showed strong enzyme inhibitory activity, as shown in Table 2.
[0069] Table 2
[0070]
[0071] IC of the remaining 84 compounds 50 Value >100nM.
Embodiment 3
[0072] Example 3: Determination of anti-cancer activity in vitro.
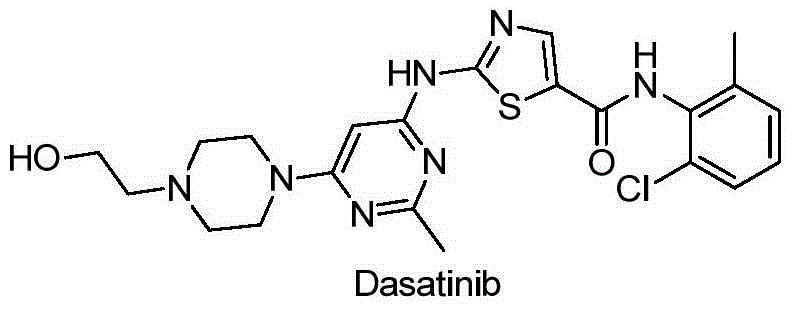
[0073] The above-mentioned 15 compounds with strong enzyme inhibitory activity were tested for their inhibitory activity on tumor cell lines by MTT method, and Dasatinib was used as a positive control drug. For specific experimental steps, refer to the literature Modern Experimental Methods in Pharmacology [M]. Beijing: Peking Union Medical College and Beijing Medical University Press, 1998: 818. The tumor cells used are: Hun78T lymphocytic leukemia cells, A549 human lung cancer cells, PC3 human prostate cells, MDA-MB-435 human breast cancer cells, HT-29 human colon cancer cells and BGC-823 human gastric cancer cells. According to the BLLIS method, the concentration IC of the drug required for the half inhibition rate of cell proliferation was calculated. 50 . See Table 3 for specific data.
[0074] table 3
[0075]
[0076] "-" means IC 50 Out of measurement range, no corresponding data obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com