Star-type macromolecular hindered phenol antioxidant and preparation method and application thereof
A technology of hindered phenols and macromolecules, used in the field of antioxidants, can solve the problems of reducing the ability to capture free radicals, unclear molecular structure, low effective antioxidant components, etc., and achieve the performance of inhibiting volatilization and migration, and resisting solvent extraction. Excellent effect with excellent thermal oxidation resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Synthesis of star-shaped macromolecule hindered phenolic antioxidant: the synthesis method is a two-step method.
[0032] The first step, add 1.222g isophorone diisocyanate (IPDI), 0.0012g dibutyltin dilaurate (DBTDL) and 5.00g chlorobenzene in the there-necked flask that is equipped with magnetic stirring, condenser, burette and nitrogen protection, Adjust the temperature to 30°C; dissolve 1.18g of 3,5-di-tert-butyl-4-hydroxymethylphenol (DBHMP) in 30.00g of chlorobenzene, add it dropwise to the reaction system within 1h, and react at constant temperature for 4h. Intermediates containing primary isocyanates are obtained.
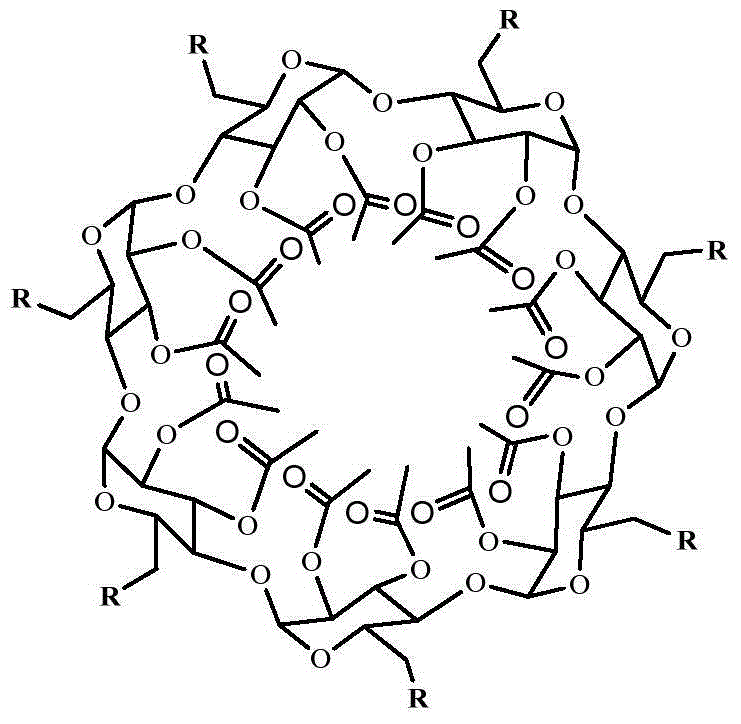
[0033]In the second step, the temperature is raised to 70°C, and 0.0024g dibutyltin dilaurate and 0.861g polyhydroxyl initiator (β-CD-core) (synthesized according to the literature: Gou PF, Zhu WP, Xu N, Shen ZQ.Synthesis and characterization of well‐defined cyclodextrin‐centered seven‐arm star poly(e‐caprolactone)s and amphiphilic star poly(e‐c...
Embodiment 2
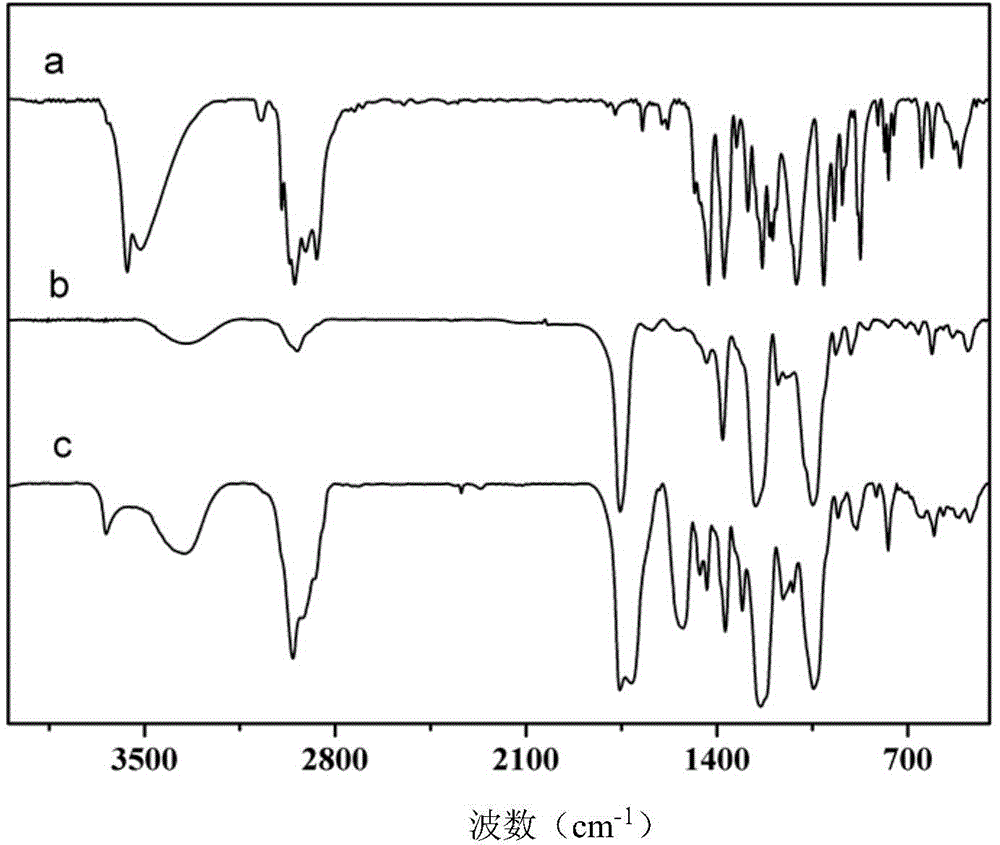
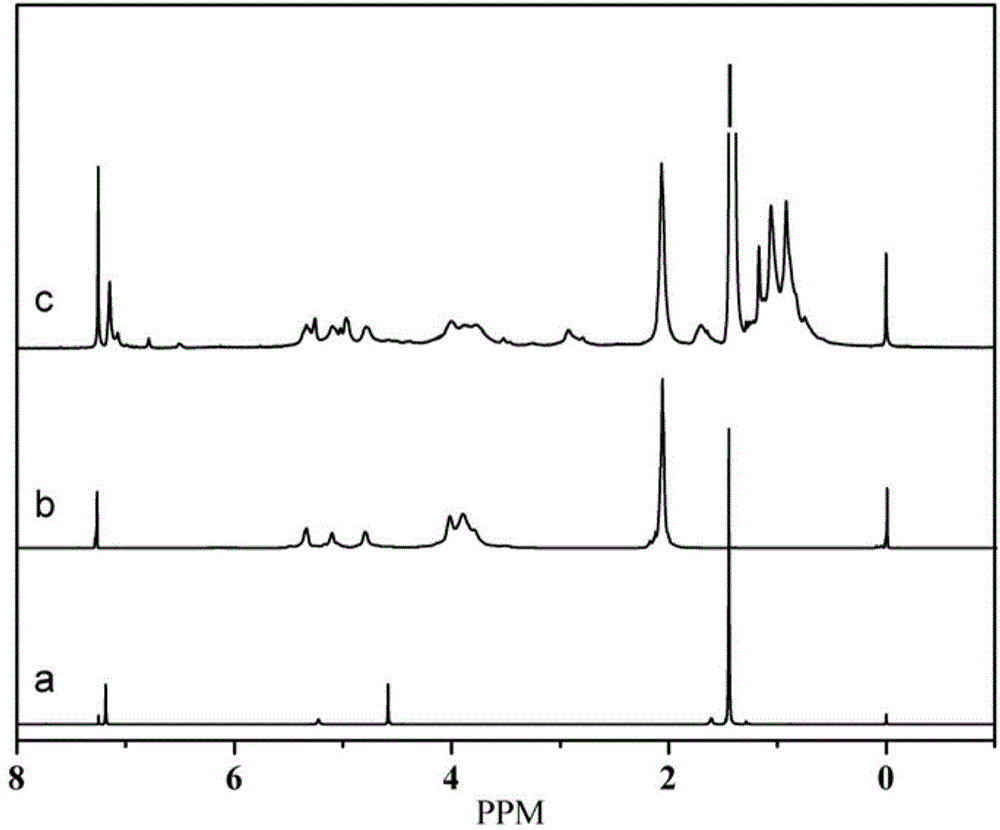
[0038] The difference between this embodiment and Example 1 is that in the first step, the mol ratio of isophorone diisocyanate and 3,5-di-tert-butyl-4-hydroxymethylphenol is changed to 1.15:1 , that is, the amount of isophorone diisocyanate is changed to 1.278g; the amount of dibutyltin dilaurate is changed to 0.0019g, the constant temperature reaction temperature is 25°C, and the amount of dibutyltin dilaurate in the second step is changed to 0.0026g . FT‐IR and 1 H‐NMR is basically the same as figure 1 , 2 , indicating the successful preparation of star macromolecule hindered phenolic antioxidants.
[0039] The thermo-oxidative aging resistance and extraction resistance of the vulcanizate are shown in Table 1. It can be seen from Table 1 that the k value of natural rubber vulcanizate is 0.51 after thermal oxygen accelerated aging at 100°C for 48 hours; after soaking in 70°C water for 48 hours, and then 100°C thermal oxygen accelerated aging for 48 hours, the k value dro...
Embodiment 3
[0041] The difference between this embodiment and Example 1 is that in the first step, the mol ratio of isophorone diisocyanate and 3,5-di-tert-butyl-4-hydroxymethylphenol is changed to 1.05:1 , that is, the amount of isophorone diisocyanate was changed to 1.167g; the amount of dibutyltin dilaurate was changed to 0.0006g, and the constant temperature reaction temperature was 35°C. The consumption of the dibutyltin dilaurate in the second step is changed into 0.0022g. FT‐IR and 1 H‐NMR is basically the same as figure 1 , 2 , indicating the successful preparation of star macromolecule hindered phenolic antioxidants.
[0042] The thermo-oxidative aging resistance and extraction resistance of the vulcanizate are shown in Table 1. It can be seen from Table 1 that the k value of natural rubber vulcanizate is 0.48 after thermal oxygen accelerated aging at 100°C for 48 hours; after soaking in 70°C water for 48 hours, and then 100°C thermal oxygen accelerated aging for 48 hours, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com