Method for preparing dialdehyde substance employing environment-friendly efficient oxidation of annular ortho diol and device required for realizing method
A technology of ortho diol and dialdehyde, which is applied in the field of fine chemical synthesis and green chemical preparation, can solve the problems of low electrolysis efficiency, high energy consumption, and low yield, and achieve simple procedures, low energy consumption, and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
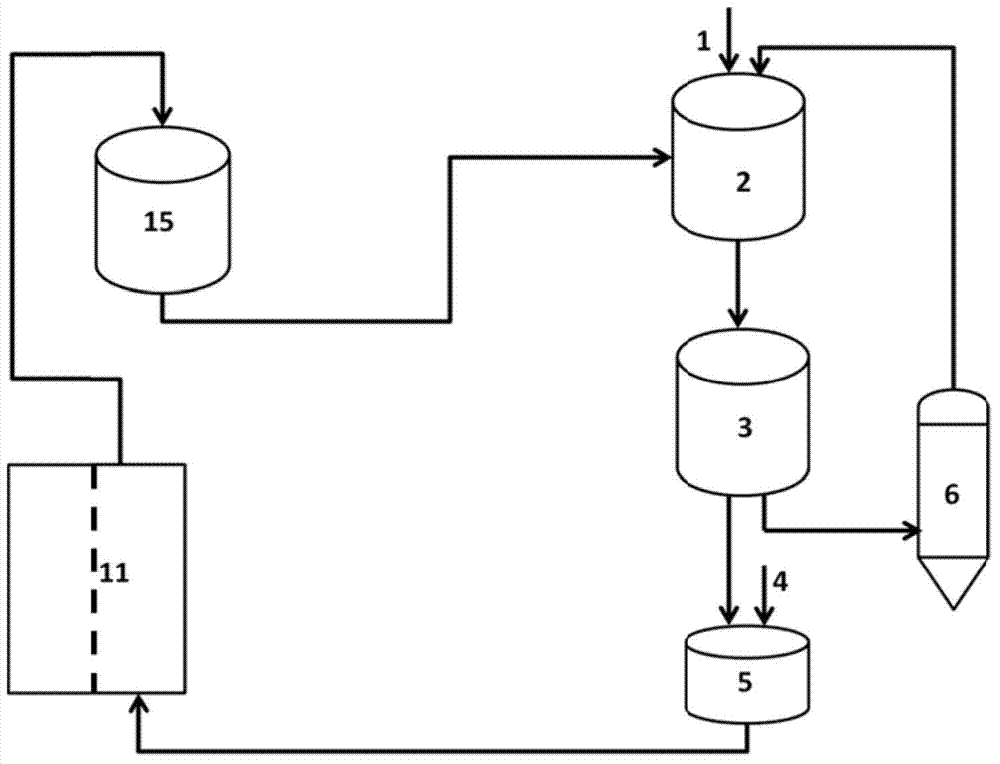
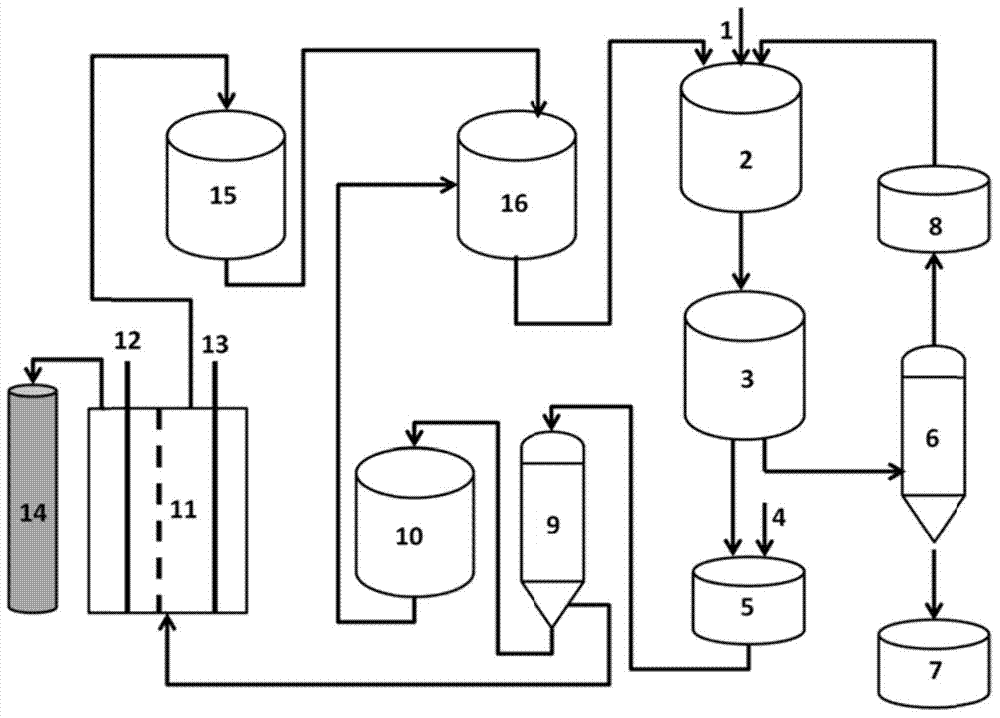
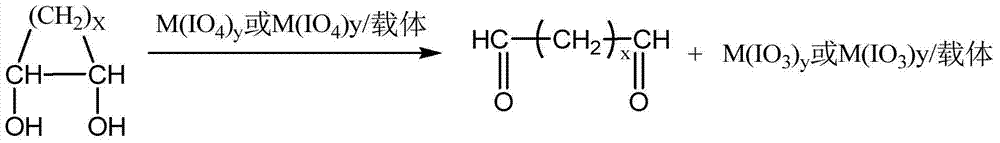
[0071] Such as figure 2 In the shown reaction device, 10 g of 1,2-cyclohexanediol, 20 mL of dichloromethane, and the ground oxidant system (10 g of sodium periodate: 10 g of silicon dioxide = 1:1, wherein the silicon dioxide is 300 mesh ) into the reactor, keep the temperature at 38°C, and stir at 600r / min, so that sodium periodate is highly dispersed in the reaction system, and then reflux dichloromethane for 2h. After the reaction is finished, the solids of the solution and the oxidant-containing system are separated by filtration. After the filtrate was distilled under reduced pressure, it was detected by gas chromatography-mass spectrometer that the purity of adipaldehyde reached 99.8%, the mass of adipaldehyde was 9.82g, and the yield was 99.72%.
[0072] Add 100g of water to dissolve the solids in the oxidant-containing system to obtain a mixture of sodium periodate, sodium iodate and silicon dioxide, filter the water-insoluble silicon dioxide and dry it to enter the n...
Embodiment 2
[0074] Such as figure 2 In the reaction device shown, 10g of 1,2-cyclohexanediol, 30mL of 1,2-dichloroethane, and the oxidant system after grinding (15g of potassium periodate: 30g of silicon dioxide = 1:2, where two Silicon oxide (600 mesh) was added into the reactor, the temperature was kept at 82°C, and stirring was performed at 150 r / min to make potassium periodate highly dispersed in the reaction system, and 1,2-dichloroethane was refluxed for 2 hours. After the reaction is finished, the solids of the solution and the oxidant-containing system are separated by filtration. After the filtrate was distilled under reduced pressure, it was detected by gas chromatography-mass spectrometer that the purity of adipaldehyde reached 99.7%, the mass of adipaldehyde was 14.7g, and the yield was 99.92%.
[0075] Add 100g of aqueous solution to dissolve the solid oxidant to obtain a mixture of potassium periodate, potassium iodate and silicon dioxide, filter the water-insoluble silico...
Embodiment 3
[0077] Add 20g of 1,2-cyclopentanediol, 30mL of ethyl acetate, and the ground oxidant system (sodium periodate 20g, without carrier) into the reactor, keep the temperature at 75°C, and stir at 300r / min to make the sodium periodate Highly dispersed in the reaction system, reflux ethyl acetate reaction for 5h. After the reaction is finished, the solids of the solution and the oxidant-containing system are separated by filtration. After the filtrate was distilled under reduced pressure, it was detected by gas chromatography-mass spectrometer that the glutaraldehyde had a purity of 99.8%, a mass of 19.61 g, and a yield of 99.97%.
[0078] Add 150g of 1M aqueous sodium hydroxide solution to dissolve the solid oxidizing agent to obtain a mixture of sodium periodate and sodium iodate, and enter the next cycle for subsequent use. Pour the aqueous solution containing sodium periodate and sodium iodate into the electrolytic cell. Add 100g / L NaCl and 0.5M HCl solution to the electrolyt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com