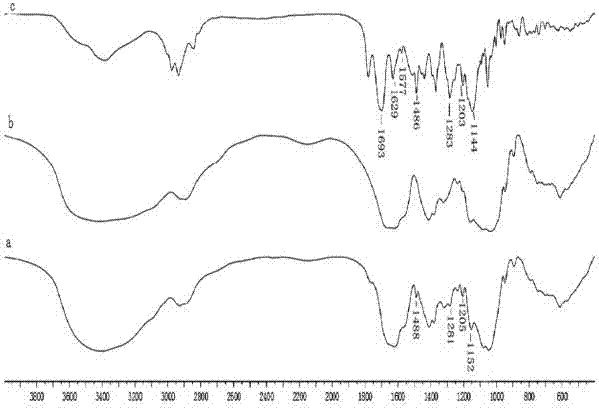
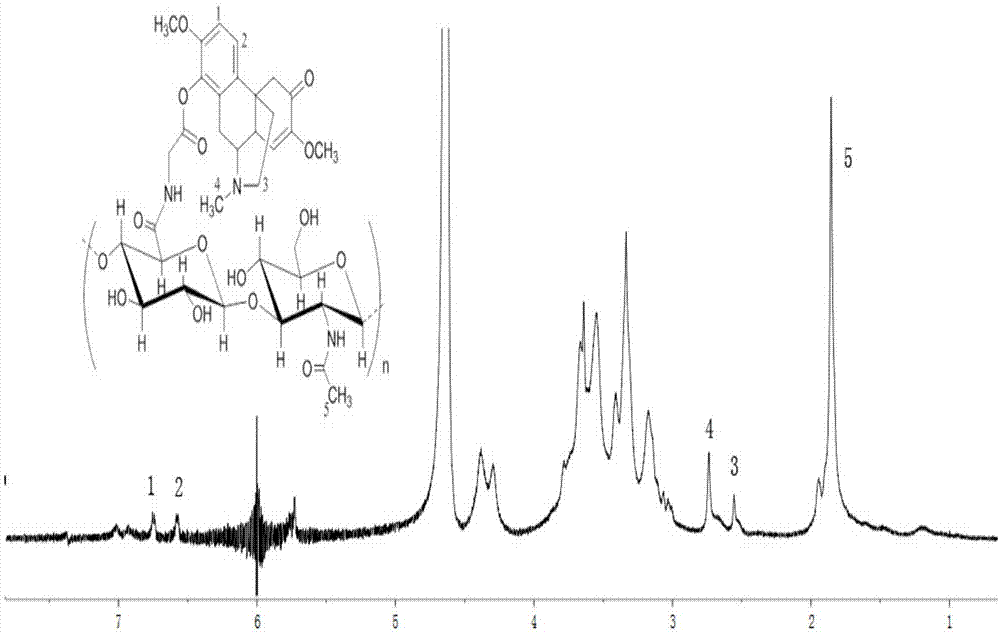
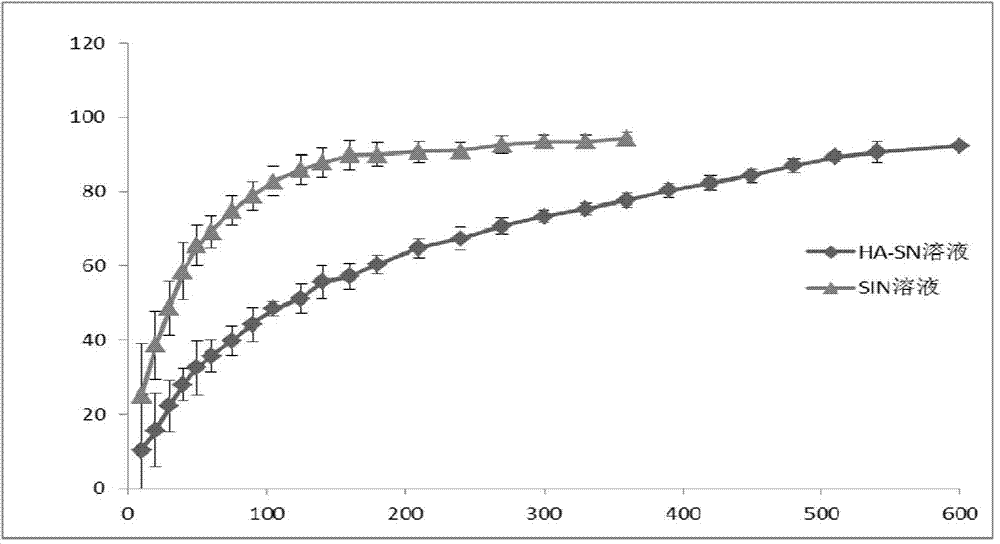
Hyaluronic acid derivative and therapeutic thereof
A technology of hyaluronic acid and its derivatives, which is applied in the field of medicine, can solve the problems of compound-related uses that have not been seen in literature and patent reports, and achieve the effects of long duration of drug effect, long retention time, and enhanced therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Preparation of sinomenine-glycine-hyaluronic acid derivatives by hyaluronic acid tetrabutylammonium salt
[0025] (1) Preparation of N-tert-butoxycarbonylglycine (Boc-Gly): 2.3 g of glycine was weighed and dissolved in 20 ml of methanol. Weigh 6.96 g of di-tert-butyl dicarbonate (Boc anhydride) and dissolve it in 4.5 ml of methanol. Add the Boc anhydride methanol solution dropwise to the glycine solution under an ice bath, and simultaneously add 7 ml of 4M sodium hydroxide solution dropwise, and react at room temperature for 2 ~5h. TCL detection reaction to complete. Add 10ml of toluene and 3ml of concentrated hydrochloric acid, add an appropriate amount of ethyl acetate for extraction, add an appropriate amount of saturated brine to the organic layer, wash twice, remove the organic solvent by rotary evaporation, and obtain a colorless oil, add ethyl acetate-toluene for recrystallization, and obtain white needles. The solid was 4.48g, and the yield was 83.6...
Embodiment 2
[0033] Example 2: Preparation of sinomenine-glycine hyaluronic acid derivatives from hyaluronic acid sodium salt
[0034] Gly-SN was prepared by the same method as (1) and (2) in Example 1. Dissolve 1.50g HA (30W) in 80ml of water completely, add 0.85g of intermediate Gly-SN, 0.85g of EDCI, 0.52g of NHS, stir to dissolve and keep the pH between 4.2-5, react at room temperature for 20h, transfer the reaction solution to the dialysis bag , 0.9% NaCl dialyzed overnight, 5 times ethanol precipitation of the dialysate to obtain a white powder. The molar ratio of the 2-sugar units of the product hyaluronic acid to the molar ratio of sinomenine is 10:1.
Embodiment 3
[0035] Example 3: Preparation of sinomenine-aspartic acid-hyaluronic acid derivatives by hyaluronic acid tetrabutylammonium salt
[0036] By a method similar to that of Example 1, substituting aspartic acid for glycine, 3.1 g of the target compound powder was obtained. The molar ratio of the 2-sugar units of the product hyaluronic acid to the molar ratio of sinomenine is 6:1.
[0037] The structure of the product is shown in the following formula.
[0038]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com