Animal bifidobacterium and composition thereof
A technology of animal bifidobacterium and composition, applied in the direction of drug combination, bacteria, microorganisms, etc., can solve the problems of unsolved room temperature storage, easy inactivation, etc., and achieve good synergistic growth, high number of viable bacteria, and good inhibition Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The acquisition of embodiment 1 bacterial strain
[0026] Bifidobacterium animalis, isolated from healthy human intestines in Jianggan District, Hangzhou City, and screened out strains suitable for human body, Bifidobacterium animalis has been preserved in the General Microbiology Center of Institute of Microbiology, Chinese Academy of Sciences on December 29, 2010, with the preservation number It is CGMCC No.4521.
[0027] Bifidobacterium animalis was collected from 20 healthy children aged 2-6 years old in Jianggan District, Hangzhou City, 10 males and 10 males, all of whom had no history of gastrointestinal diseases and did not take any antibacterial drugs within two weeks before sampling. When sampling, use a sterile glass rod to pick up 4-10g of naturally discharged fresh feces, place them in a sterile plate, quickly put them into an anaerobic tank, and bring them back to the laboratory for separation.
Embodiment 2
[0028] Identification and cultivation of embodiment 2 bacterial strains
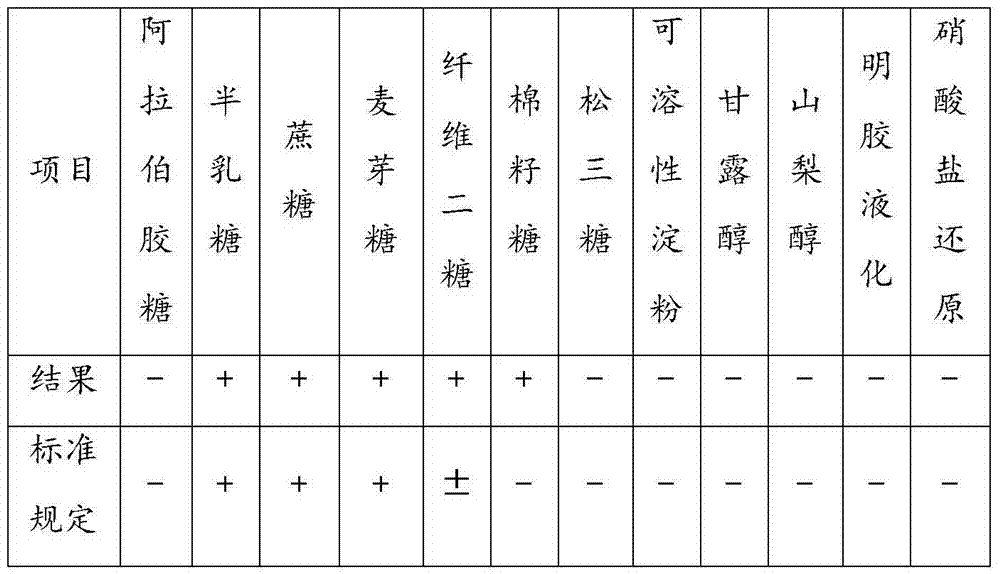
[0029] 2.1 Use the dilution coating method to separate and purify the collected strain samples. After purification and culture, observe the colony morphology, take fresh culture, inoculate sugar fermentation test tube, and identify the biochemical reaction characteristics of the strain. At the same time, take the fresh culture of the strain to be tested, use the API 20A biochemical identification strip of the French Mérieux company, and refer to the biochemical identification database of the Mérieux company for API biochemical identification of the strain to be tested. At the same time, the 16S rRNA of the isolated and purified strain will be extracted and sequenced, and the sequencing results will be compared with the existing sequences in NCBI's GeneBank. The isolated and purified strains were stored at 2-8°C.
[0030] After identification, the colonies of the strains to be tested are characterized b...
Embodiment 3
[0049] Example 3 Stomach Acid Resistance and Bile Salt Test of Bacterial Strains
[0050] 3.1 Bile salt tolerance test
[0051] The composition of the improved TPY nutrient solution used for cultivating bacterial strains is:
[0052] The isolated and purified bacterial strains were cultured strictly anaerobically at 37° C. for 15 h with sterilized modified TPY medium. The activated strain culture solution was inserted into the aseptic modified TPY liquid medium containing different bile salt concentrations with 10% inoculum (the bile salt concentration was 1%, 2%, 3%, 4%, 5%, 6%, 7% ), while the improved TPY medium without bile salts was used as a control. After anaerobic culture at 37°C for 0, 6, 12, 18, and 24 hours, samples were taken to determine the number of viable bacteria.
[0053] 3.2 Acid tolerance test
[0054]The isolated strains were cultured strictly anaerobically at 37° C. for 15 h with sterile modified TPY medium. The culture solution of the activated stra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com