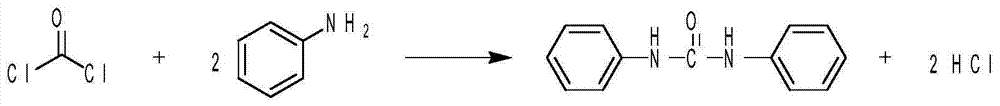
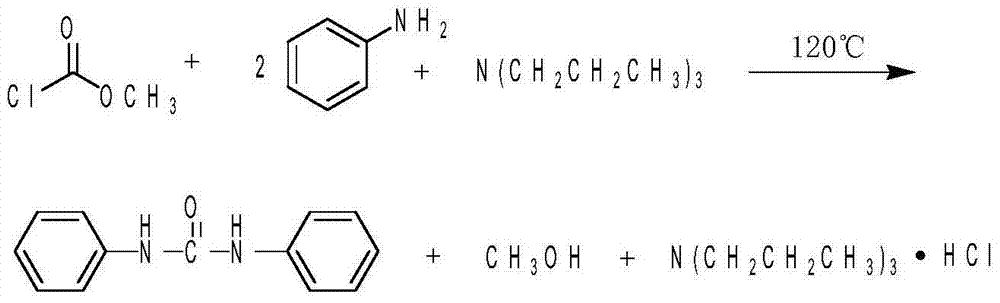
Method for synthesizing diphenyl urea and derivative of diphenyl urea
A technology of diphenylurea and synthesis method, which is applied in the preparation of urea derivatives, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of low yield, heavy pollution and high temperature, and achieve high utilization rate of raw materials, Good product quality and less pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0031] Example 1 is the methyl chloroformate method reported in the literature. Add aniline 9.32g and tri-n-butylamine 27.75g successively in a 250mL round-bottomed flask, add methyl chloroformate 14.18g dropwise under ice-water cooling and stirring, and heat to 120 Make it reflux at ℃, react for 3 hours, cool to room temperature, add 50mL of absolute ethanol, and slowly pour into 250mL of distilled water under stirring, forming a khaki flocculent precipitate. Stand overnight at 4°C, filter with suction the next day, and wash the precipitate with water three times. Recrystallized twice from absolute ethanol and dried at 100°C for 2 hours to obtain a solid with a yield of 90.50%.
example 2
[0033] Add 30.00g of dichloromethane, 1.18g of triethylamine, and 0.99g of aniline to a 100mL four-neck flask in sequence. According to the molar ratio of aniline to benzyl chloroformate is 1.0:1.1, add benzyl chloroformate dropwise under stirring at 0-10°C Esters 2.00g, continue to stir at room temperature for 1h after the dropwise addition is completed, add an equal amount of ethyl acetate as a solvent to the reaction solution, stir, filter to remove triethylamine hydrochloride, add saturated aqueous sodium bicarbonate solution to the filtrate, and stir for 15min , standing for stratification, extraction, washing with water for 3 to 5 times, taking the organic layer, adding anhydrous magnesium sulfate to dry, filtering, and distilling off the organic solvent under reduced pressure to obtain a solid, which is then separated by column chromatography to obtain a product with a purity of 95.69% , and the yield was 91.67%.
example 3
[0035] Add 30.00g of dichloromethane, 1.18g of triethylamine, and 1.31g of p-methoxyaniline to a 100mL four-necked flask in sequence. Add 2.00 g of benzyl chloroformate dropwise under stirring at ℃, continue to stir at room temperature for 5 hours after the dropwise addition is completed, add an equal amount of ethyl acetate as a solvent to the reaction solution, stir, remove triethylamine hydrochloride by filtration, and add saturated Aqueous sodium bicarbonate solution, stirred for 15 minutes, allowed to stand for stratification, extracted, washed 3 to 5 times with water, took the organic layer, added anhydrous magnesium sulfate to dry, filtered, and distilled off the organic solvent under reduced pressure to obtain a solid, which was then subjected to column chromatography After separation, the purity of the product obtained was 99.63%, and the yield was 81.38%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com