A kind of azo chiral metal complex, its synthesis method and application
A technology of metal complexes and synthesis methods, applied in chemical instruments and methods, preparation of imino compounds, color-changing fluorescent materials, etc., can solve the problem of not paying attention to the relationship between photo-induced cis-trans isomerism of azobenzene, etc., and achieve good application Potential, mild reaction conditions, and the effect of readily available starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
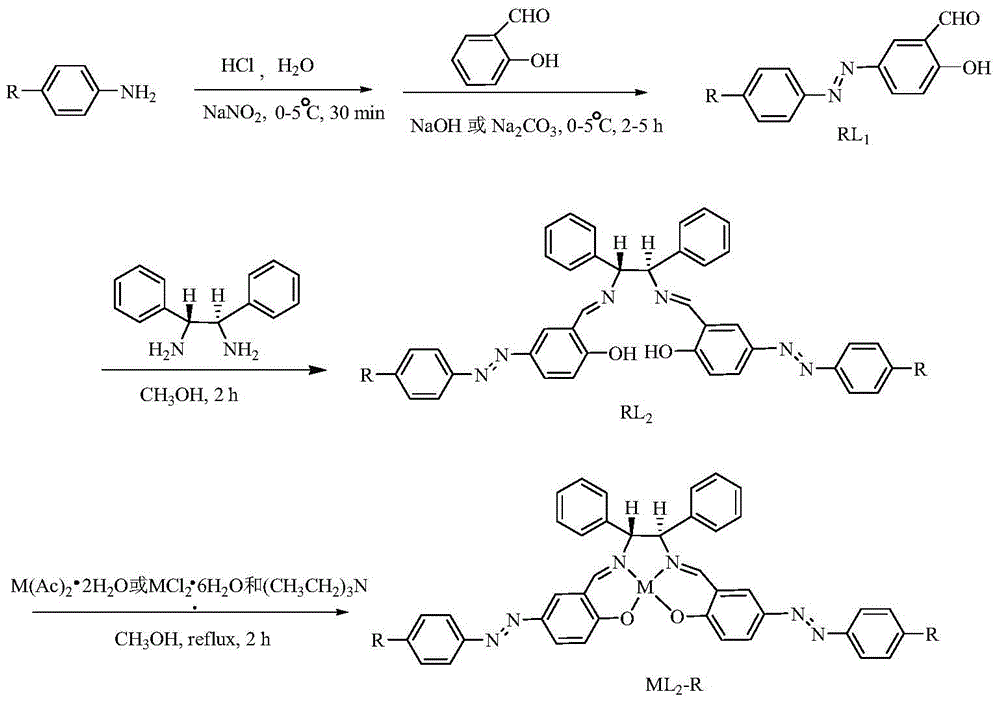
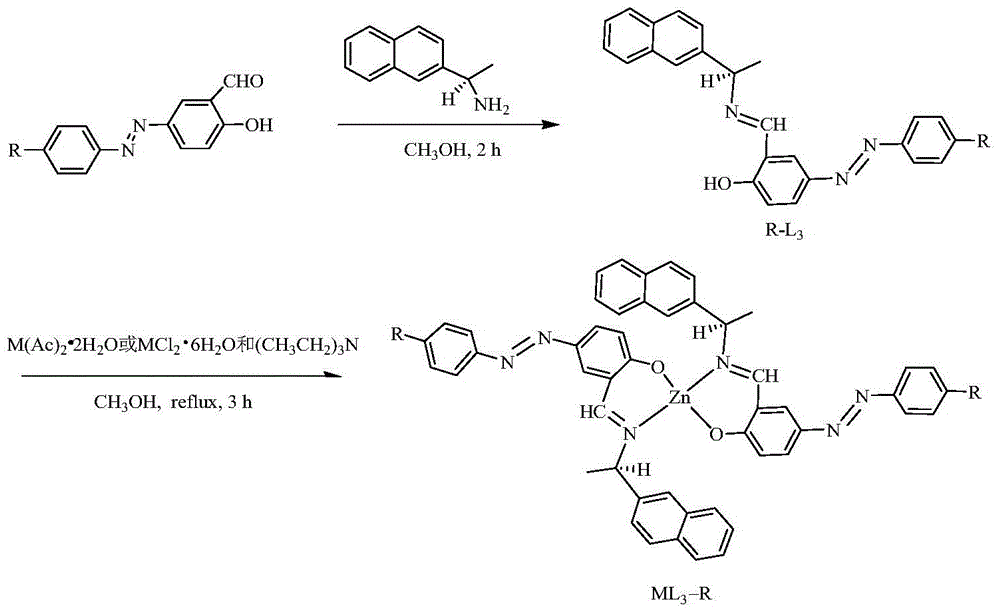
[0047] (1) Aniline (5.0mmol, 0.46g) was dissolved in 3.5mL of 5mol / L dilute hydrochloric acid solution (1.5ml of 12mol / L concentrated hydrochloric acid dissolved in 2ml of distilled water), placed in an ice bath and stirred; dropwise added 5 % sodium nitrite solution (5.5mmol, 0.38g was dissolved in 7.2ml water) until the starch potassium iodide test paper just turned blue, and continued to stir in an ice bath for half an hour to complete the diazotization reaction to obtain a diazonium salt solution; The diazonium salt solution was added dropwise to ethanol or acetonitrile solution containing 10ml salicylaldehyde (5mmol, 0.61g), and the pH was adjusted to 7-8 with an aqueous solution of sodium carbonate or sodium hydroxide. Among the present examples, hydrogen Aqueous solution of sodium oxide (5mmol, 0.02g dissolved in 2ml of water) adjust pH to 7~8, continue to react in ice bath for 2h, filter to obtain precipitate, and wash the precipitate with a mixed solvent of ethanol and...
Embodiment 2
[0062] (1) Dissolve p-methoxyaniline (5.0mmol, 0.47g) in 3.5mL of 5mol / L dilute hydrochloric acid solution (1.5ml of 12mol / L concentrated hydrochloric acid dissolved in 2ml of distilled water), place in an ice bath and stir Add dropwise 5% sodium nitrite solution (5.5mmol, 0.38g dissolved in 7.2ml water) until the starch potassium iodide test paper just turns blue, continue to stir in the ice bath for half an hour to complete the diazotization reaction, and obtain the diazonium salt solution The diazonium salt solution that will make is added dropwise in the ethanol or the acetonitrile solution that contains 10ml salicylaldehyde (5mmol, 0.61g), adjusts pH to with the aqueous solution of sodium hydroxide (5mmol, 0.02g is dissolved in the water of 2ml) 7-8, continue to react in an ice bath for 5 hours, filter to obtain a brownish-yellow precipitate, wash the precipitate with a mixed solvent of ethanol and water with a volume ratio of 1:1, and dry it in vacuum. The obtained precip...
Embodiment 3
[0077] (1) Dissolve p-nitroaniline (5.0mmol, 0.46g) in 3.5mL of 5mol / L dilute hydrochloric acid solution (1.5ml of 12mol / L concentrated hydrochloric acid dissolved in 2ml of distilled water), and stir in an ice bath; Add dropwise 5% sodium nitrite solution (5.5mmol, 0.38g dissolved in 7.2ml water) until the starch potassium iodide test paper just turns blue, and continue to stir in an ice bath for half an hour to complete the diazotization reaction to obtain a diazonium salt solution; The prepared diazonium salt solution was added dropwise to ethanol or acetonitrile solution containing 10ml of salicylaldehyde (5mmol, 0.61g), and the pH was adjusted to 7 with an aqueous solution of sodium hydroxide (5mmol, 0.02g dissolved in 2ml of water) ~8, continue to react in an ice bath for 2 hours, filter to obtain a brownish-yellow precipitate, wash the precipitate with a mixed solvent of ethanol and water with a volume ratio of 1:1, and dry it in vacuum. The obtained precipitate is the i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com