Method for recovering lithium resource from lithium-ion-containing solution by using lithium ion carrier
A lithium ion and carrier technology, applied in the field of lithium resource extraction, can solve the problems of complex process, environmental pollution, equipment corrosion, etc., and achieve the effect of reducing recovery cost, high-efficiency lithium resources, and high-efficiency recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
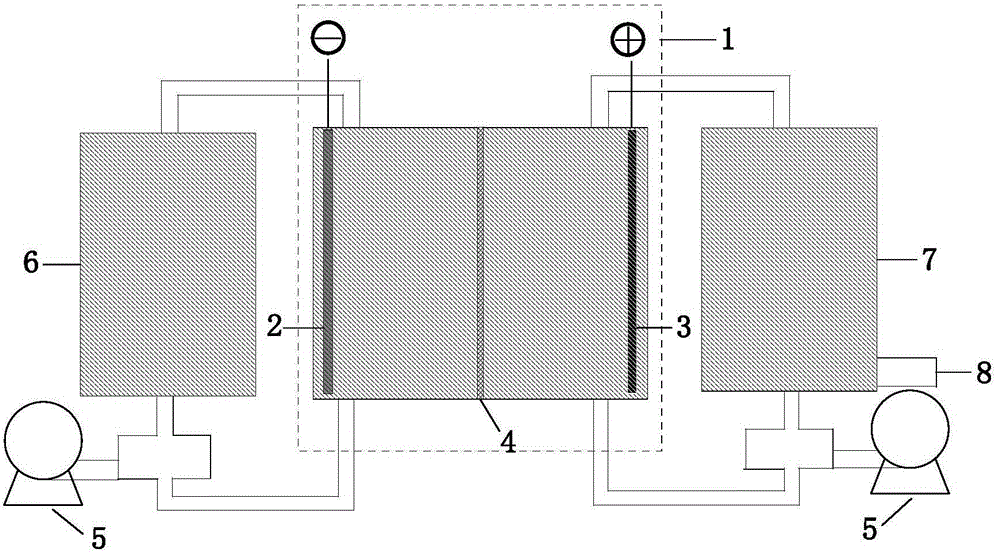
[0035] Take 100 g LiCoO 2 As a lithium ion carrier in a lithium-rich state, 316L stainless steel was made into a cylindrical shape (diameter 8cm, height 6cm), as an anode, the lithium ion carrier was placed in a straight cylinder, and magnetic stirring was used at the bottom of the reaction vessel to ensure LiCoO 2 The effective contact with the electrode is in a suspended state in the annular base, and the cathode is a foamed nickel cylindrical cathode (5 cm in diameter, 6 cm in height) separated by a separator paper. Both the anode and cathode solutions of the electrolytic cell were filled with the same 0.5mol / L LiCl solution and an external DC power supply was used to conduct electrolysis at a constant current of 1A (current density: 10mA / g) for 16 hours to make LiCoO 2 Lithium ion carrier transformed into a lithium-poor state.
[0036] The lithium ion carrier in the lithium-poor state obtained above is used as a carrier for lithium ion absorption of a salt lake brine solu...
Embodiment 2
[0041] Spinel LiMn after delithiation with pre-acidification 2 O 4 As a lithium ion carrier in a lithium-poor state, pre-acidification refers to adding 10 grams of LiMn to 2 O 4 Stir continuously in 2 liters of 0.2 mol / L HCl solution to make them fully contact, and after 12 hours of reaction, filter, wash and dry to obtain a lithium ion carrier in a lithium-poor state. (for the prior art) it is made into an electrode, and the weight percentages of the four components of the electrode are as follows:
[0042] Lithium ion carrier is spinel LiMn after pre-acidification and delithiation 2 O 4 : 51.3%;
[0043] The conductive material is expanded graphite: 8.8%;
[0044] Carrier material nickel foam: 37.8%;
[0045] Binder PTFE: 2.1%.
[0046] The counter electrode is capacitive carbon mixed in PTFE to make the auxiliary electrode. On the cathode carrier of the electrolytic cell, the above-mentioned electrode sheet containing the carrier of lithium ions in a lithium-poor s...
Embodiment 3
[0048] Commercially available LiCoO 2 It is a carrier of lithium ions in a lithium-rich state, and it is made into an electrode. The weight percentages of the four components of the electrode are as follows:
[0049] Lithium ion carrier is LiCoO 2 : 49.4%;
[0050] The conductive material is expanded graphite: 9.6%;
[0051] Carrier material nickel foam: 38.8%;
[0052] Binder PTFE: 2.2%.
[0053] The recovery process of lithium ions is as follows:
[0054] (1) Put 100 grams of LiCoO 2 The lithium ion carrier electrode prepared according to the above ratio is used as the anode, the counter electrode is the capacitive carbon mixed in PTFE to make the auxiliary electrode, and the reference electrode is the SCE electrode. 0.5 liter of 0.5 mol / L MgSO to be recovered is passed into the cathode chamber 4 and 0.5mol / L Li 2 SO 4 The mixed solution is catholyte. The anode chamber is fed with 1 liter of 1 mol / L Na 2 SO 4 and 0.005mol / L LiOH solution as anolyte.
[0055] in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com