Glutathione peroxidase GPX2 mutant containing serine and preparation method of mutant
A glutathione peroxide and mutant technology, which is applied in the biological field, can solve the problems that the catalytic group cannot achieve the specificity of the gene mutation method, is not suitable for the direct expression of selenoproteins, and the yield of simulated enzymes is decreased, etc. Achieving the effect of avoiding yield drop and inactivation, high yield and not easy inactivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of genetically engineered human GPX2 mutant protein using a synthetic gene combined with SPP and auxotrophic prokaryotic expression system
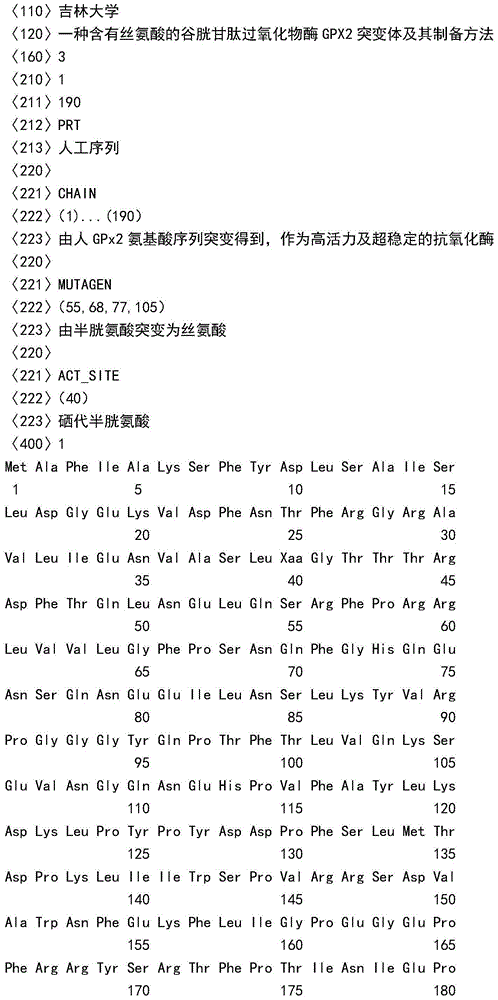
[0032] According to the amino acid sequence of the GPX2 mutant described in Sequence 1 (SEQ ID No: 1) of the present invention, artificially synthesized in a biological company with a DNA synthesizer, it can be expressed in auxotrophic strains described in Sequence 1 (SEQ ID No: 1). The gene of the GPX2 mutant protein ensures that the 5' end of the GPX2 mutant gene contains a start codon (ATG) and an Nde I restriction site, and the 3' end contains a stop codon and a Hind III restriction site, and the GPX2 mutation The coding sequence TGA of No. 40 SeCys of the body gene was replaced by the codon (TGC) of Cys, and the full length of the gene did not contain the ACA sequence; the specific target gene sequence was the No. , 68, 77 and 105 cysteine codons are replaced by serine coding sequence (in sequence: AGC...
Embodiment 2
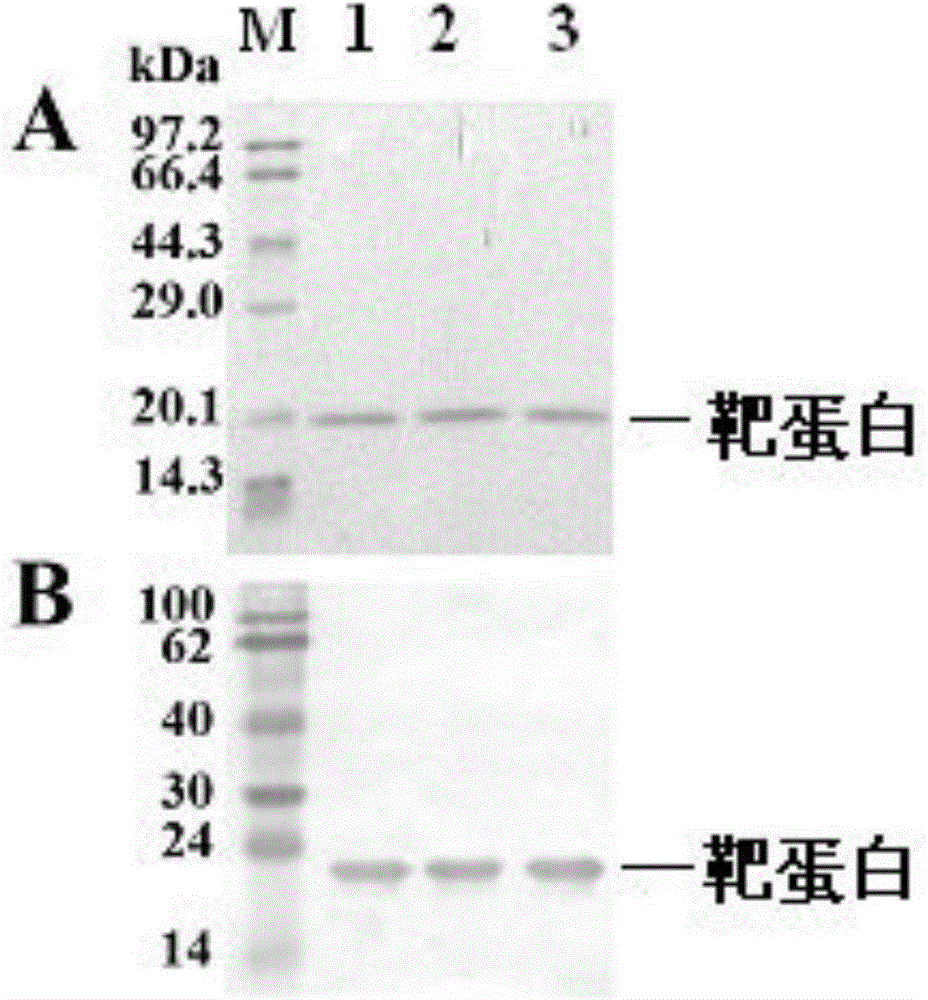
[0036] The preparation method is exactly the same as in Example 1, except that the 117th alanine coding sequence is replaced with a serine codon (TCC) when synthesizing the coding gene of the GPX2 mutant, and the 117th of the finally obtained GPX2 mutant protein is Serine, not alanine, and other amino acid sequences are completely identical to the first method, that is, the GPX2 mutant protein described in Sequence 2 (SEQ ID No: 2) of the present invention. The molecular weight of the mutant has only slight changes, and there is no difference in the results of SDS-PAGE and Western Blot, see figure 1 Lane 9 of . But its GPX activity is 2417U / μmol, slightly higher than that of Example 1, reaching the same order of magnitude as natural GPX.
Embodiment 3
[0038] Except that the expression vector pCold III (TAKARA, Cat. #3369) used in Example 1 was replaced with pCold I (TAKARA, Cat. #3367) with a histidine purification tag, the rest were exactly the same as in Example 1, that is, the final The GPX2 mutant protein described in Sequence 3 (SEQ ID No: 3) of the present invention was obtained. The mutant introduces 16 exogenous amino acids including 6 histidines on the carrier at the amino terminal, so the molecular weight has increased, which can be seen on the results of SDS-PAGE and Western Blot, see figure 1 The advantage of lane 11 is that the target protein can also be purified with a nickel-affinity layer system. Its GPX activity is 2336U / μmol, which is slightly lower than Example 2, which proves that the purification tag has no obvious effect on the activity of the protein, and the activity reaches the same order of magnitude as natural GPX.
[0039]
[0040]
[0041]
[0042]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com