Proteome sample pretreatment method based on novel nanometer composite material, and applications thereof
A nanocomposite material and sample pretreatment technology, applied in the field of preparation of nano-gold modified graphene oxide nanocomposites, can solve the problems of low-abundance protein masking, long running time, cumbersome operation, etc., and achieve good reproducibility, Reduced complexity, particle stabilization effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. Material Characterization
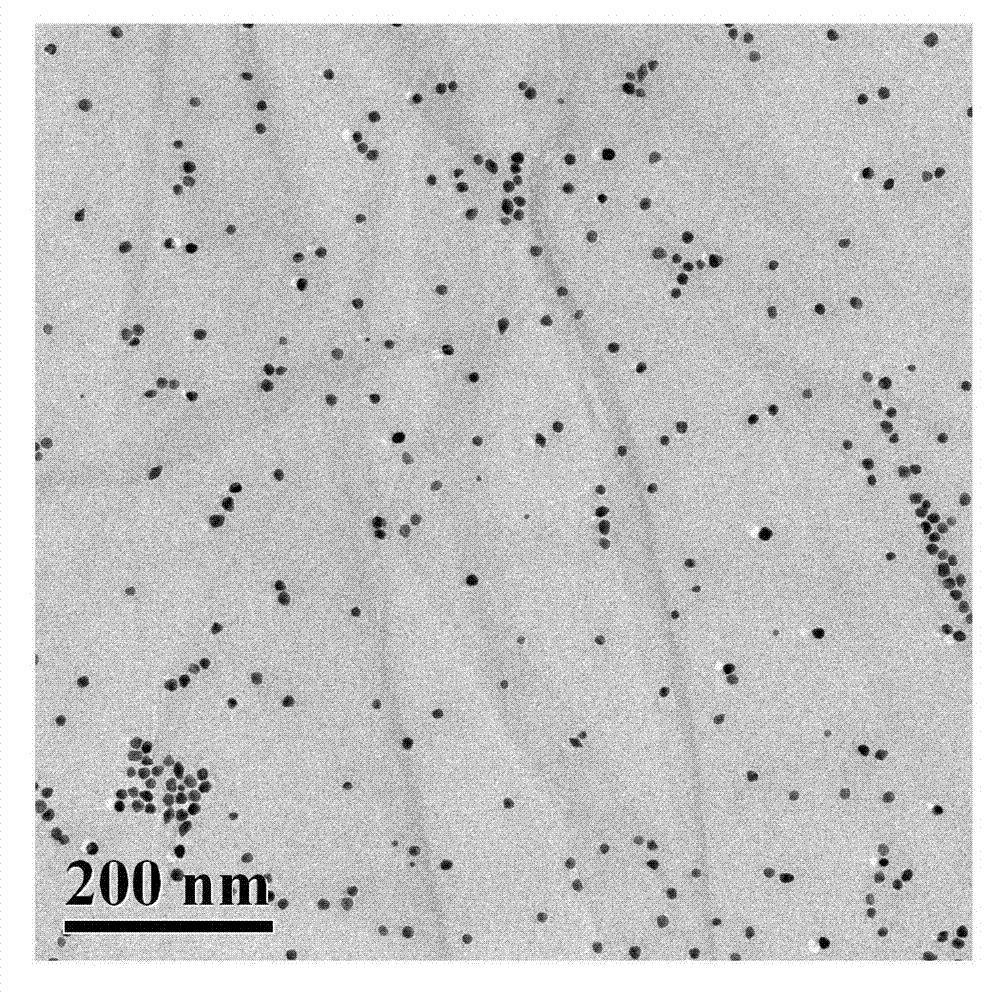
[0023] The prepared polyethyleneimine-coupled nano-gold modified graphene oxide nanocomposite particles were characterized by transmission electron microscopy as follows: figure 1 As shown, according to this figure, it can be seen that the nano-gold particles are uniformly dispersed in the graphene layer, indicating that the modified polyethyleneimine on the graphene layer successfully reduces the chloroauric acid solution, generates nano-gold particles on its surface, and the material Has very good dispersibility.
[0024] 2. Sample handling process
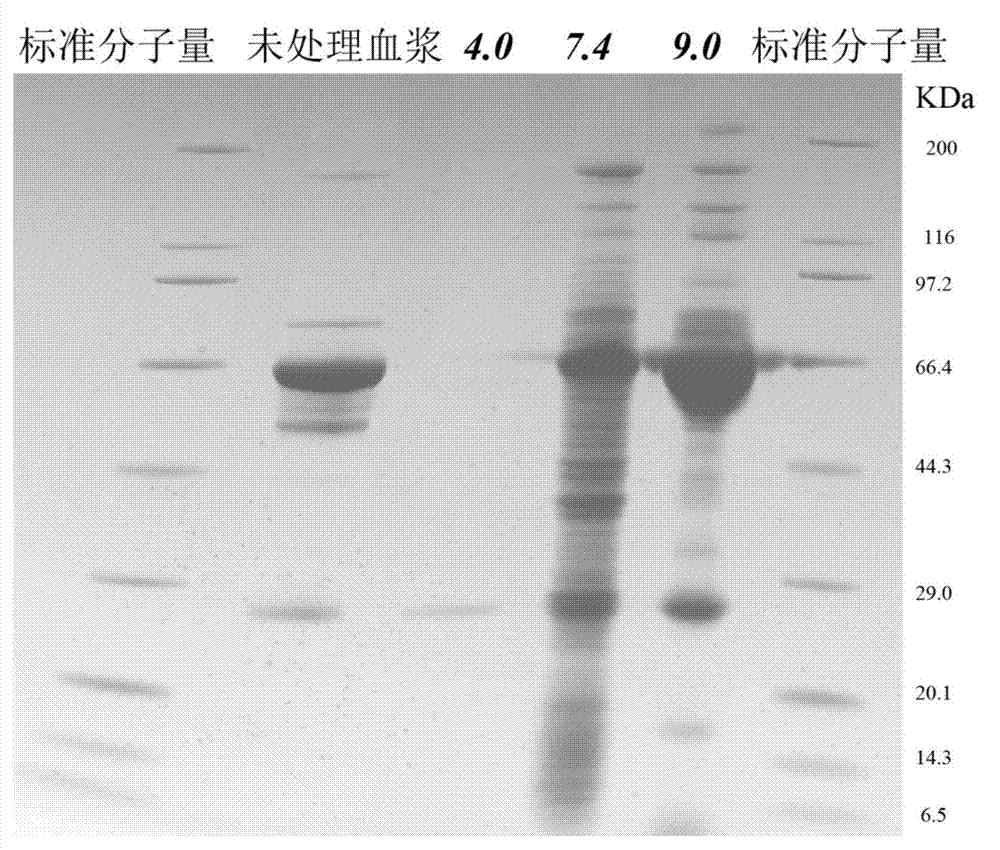
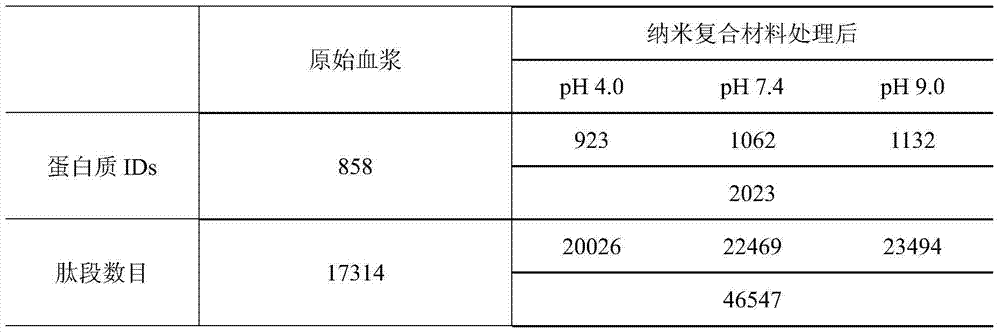
[0025] The prepared nanocomposite particles were incubated with plasma protein samples in three phosphate buffer solutions with pH values of 4.0, 7.4, and 9.0 for 0.5 h, and the incubation temperature was 25°C. After the particles are centrifuged to remove the supernatant, the phosphate buffer with the same pH value as the incubation solution is used for washing, and the supernatant is r...
Embodiment 2
[0028] 1. Enzymatic hydrolysis of proteins and nanocomposites
[0029] The compound of the obtained nano-composite material and protein was heated and denatured, and then trypsinized respectively. The specific process is as follows: Denature the sample at 90°C for 20 minutes, add a certain amount of dithiothreitol (DTT, add 8 μmol of dithiothreitol solution per mg of protein), and react at 56°C for 1.5 hours to achieve protein reduction. Process, then add iodoacetamide solution (IAA, 1 μmol DTT adds 2.5 μmol IAA) in proportion, react 40min under dark conditions to carry out alkylation to protein, finally add trypsin (the mass ratio of trypsin and protein is 1: 25), react at 37°C for 16h.
[0030] 2. Ion exchange-reversed phase tandem liquid mass analysis and data processing
[0031]After centrifuging the material after the above enzymatic hydrolysis, the supernatant was collected and analyzed by ion exchange-reverse phase tandem liquid mass (SCX-RPLC-MS / MS). Three mobile ph...
Embodiment 3
[0036] The prepared nanocomposite material was incubated with mouse brain tissue protein samples in citric acid buffer solution of pH 3.0, sodium acetate buffer solution of pH 9.0 and phosphate buffer solution of pH 7.0, and the tissue samples were processed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com