CALB mutant used for preparing (R)-3-substituted monoalkyl glutarate compound through non-aqueous catalysis
A mutant and substrate technology, applied in the field of bioengineering, can solve the problem of low selectivity of R-type products, and achieve the effects of high atom utilization, high optical purity and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
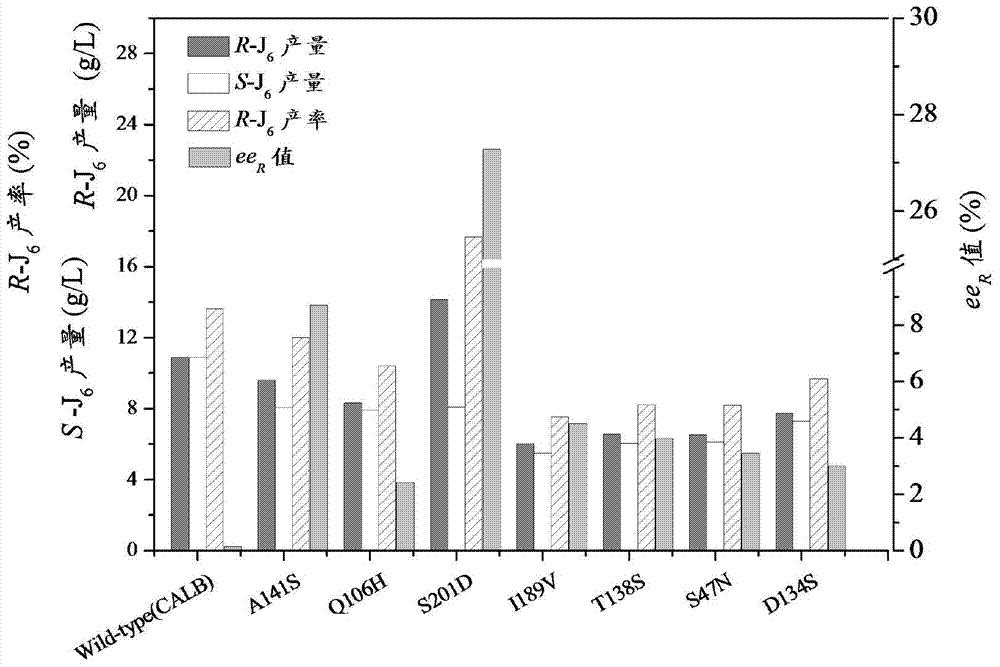
[0049] Put the airtight 10ml shaking flask into a constant temperature shaker, add 3-tert-butyldimethylsilyl glutaric anhydride (substrate) 80g / L, the molar concentration ratio of methanol and substrate is 3:1, biological The catalyst is a CALB mutant, mainly including A141S, Q106H, S201D, I189V, T138S, S47N, D134S, and the mass of biological free enzyme added is 0.60g. After adding, fix the temperature at 25°C and react for 24 hours. Chromatographic monitoring, after the reaction is completed, post-treatment, removal of organic solvent and unreacted methanol, purification treatment to obtain colorless crystal (R)-3-tert-butyldimethylsilyl glutaric acid monomethyl ester.
[0050] Experimental results such as figure 1 , compared with the control group, all mutants ee R The values were all increased, and the effect of A141S and S201D mutants was the most obvious. A141S mutant ee R Value up to 8.7%, (R)-3-tert-butyldimethylsilyl glutaric acid monomethyl ester (R-J 6 ) yield...
Embodiment 2
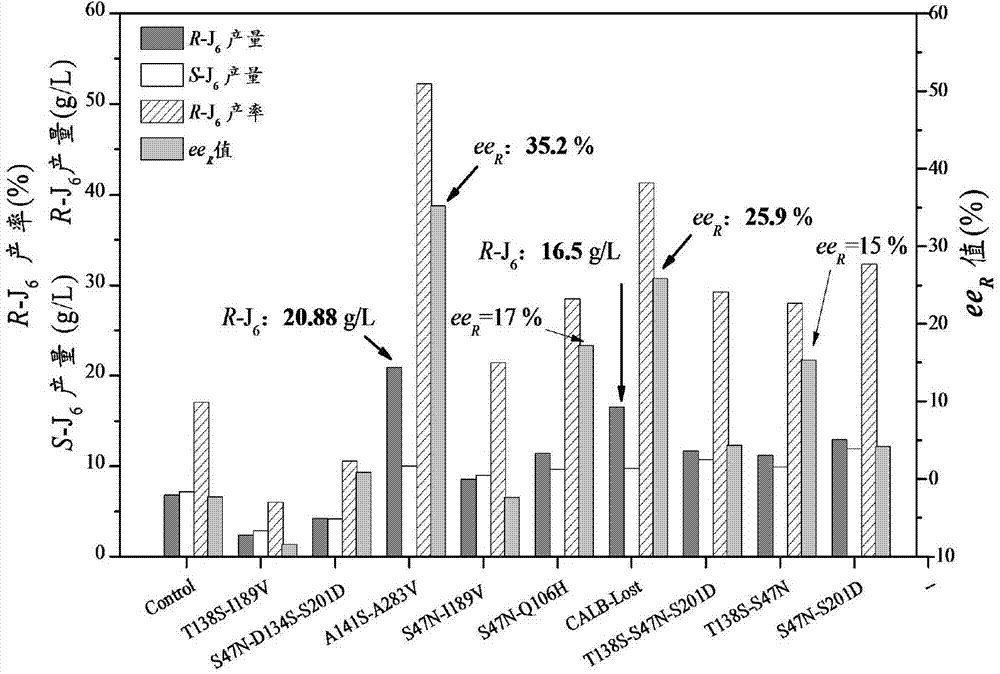
[0052] Put the airtight 10ml shaker flask into a constant temperature shaker, add 3-tert-butyldimethylsilyl glutaric anhydride (substrate) 40g / L, the molar concentration ratio of methanol and substrate is 3:1, biological The catalyst is a CALB mutant, including T138S-I189V, S47N-D134S-S201D, A141S-A283V, S47N-I189V, S47N-Q106H, CALB-Lost, T138S-S47N-S201D, T138S-S47N, S47N-S201D, biological free enzyme The added mass is 0.60g. After the addition is completed, the temperature is fixed at 25°C and reacted for 24 hours. During the period, it is monitored by liquid chromatography. After the reaction is completed, the post-treatment removes the organic solvent and unreacted methanol, and purifies to obtain colorless crystals. (R)-3-tert-Butyldimethylsilyl glutaric acid monomethyl ester.
[0053] Enzyme-catalyzed synthesis of R-J by multi-site mutants of CALB gene 6 The effect is as figure 2 , A141S-A283V and CALB-Lost mutants are more effective in catalyzing the synthesis of R-t...
Embodiment 3
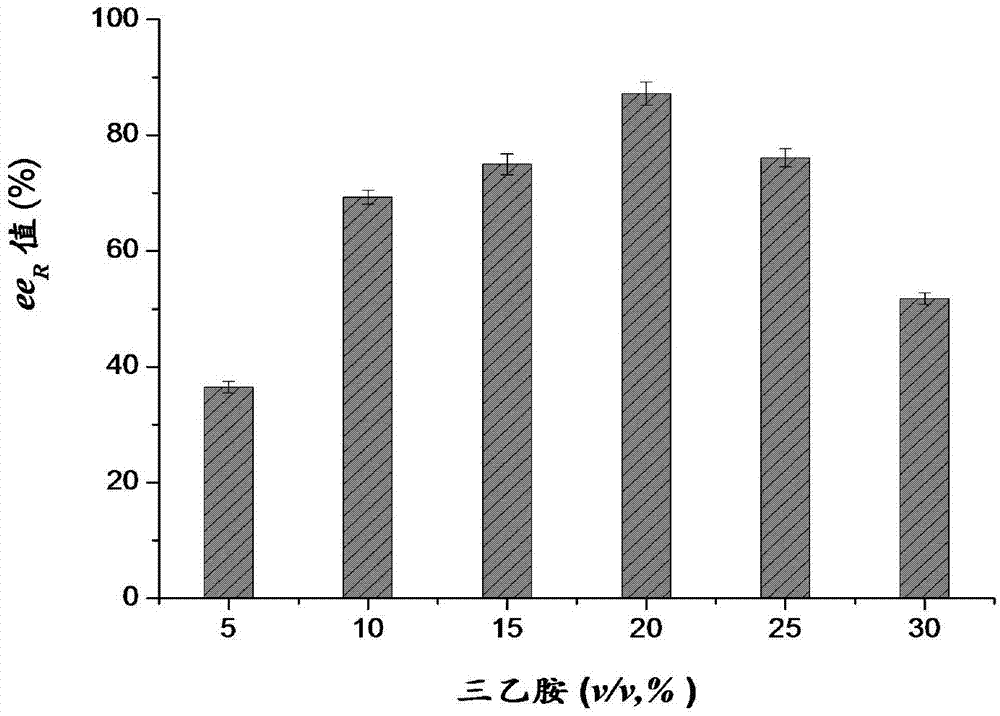
[0055] Put the airtight 10ml shaking flask into a constant temperature shaker, add 3-tert-butyldimethylsilyl glutaric anhydride (substrate) 80g / L, the molar concentration ratio of methanol and substrate is 3:1, biological Catalyst is A141S-A283V mutant, the quality that biological free enzyme adds is 0.60g, adds the triethylamine (organic base) of different concentration (5-30% v / v) in the system, investigates the effect of organic base on esterification reaction ee R It is worthy of influence, the addition is completed, and the reaction is carried out for 24 hours. During the period, it is monitored by liquid chromatography. After the reaction is completed, the post-treatment is performed to remove the organic solvent and unreacted methanol, and the purification process obtains colorless crystal (R)-3-tert-butyl dimethyl Monomethyl silyl glutarate. A141S-A283V Catalyzed Synthesis of R-J by Adding Different Volumes of Triethylamine 6 The impact of the results such as image...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com