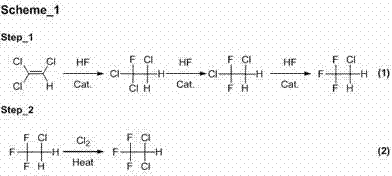
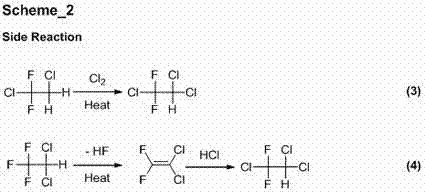
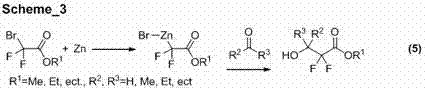
Preparation method of 2-bromo-2,2-difluoroacetyl chloride and 2-bromo-2,2-difluoro acetate and recycling method of waste difluoro trichloroethane
A technology of difluorotrichloroethane and difluorodichlorodibromoethane, which is applied in the field of preparation of fluorine-containing organic intermediates, can solve the problems of post-processing difficulties, difficulties, and many amplification problems, so as to reduce environmental pollution and Hazards to the health of operators, the effect of realizing recycling and reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0078] Install a 5-liter three-neck flask with a reflux condenser, a mechanical stirring device, and a constant pressure dropping funnel. During the reaction, control the temperature of the cooling water in the reflux condenser at 0 to 30°C, and add waste difluorotrifluorotrifluoride to the constant pressure dropping funnel. Ethyl chloride (R122, content 90.3%) 1872 grams, add 1500 grams of dehydrated alcohol in reaction flask, 467 grams of potassium hydroxide, heating and dissolving, be mixed with the potassium hydroxide solution of ethanol, adopt oil bath heating reaction flask, adopt The electronic temperature controller controls the reaction temperature to 80±5°C, and R122 is added dropwise under stirring, and the drop is completed in about 7 hours, and then the reaction is kept for 2 hours. Add 450 milliliters of solvent dichloromethane and 1373 grams (440 milliliters) of bromine into the second 5-liter three-necked flask equipped with a reflux condenser and a mechanical s...
Embodiment 12
[0103] Example 12 (purification of 2-bromo-2,2-difluoroacetyl chloride by rectification)
[0104] Install a rectification column, a fractionating head with an adjustable reflux ratio-reflux condenser, and a 2-liter three-neck flask (rectification kettle) with magnetic stirring, add 3012 grams of crude 2-bromo-2,2-difluoroacetyl chloride, During rectification, control the circulation temperature of the cooling water in the reflux condenser to be between 0 and 10°C, connect the receiving bottle of the product, use an oil bath to heat the reaction bottle, and use an electronic temperature controller to control the temperature of the oil bath to 75 to 80°C. The distillate between 49-51 ℃ was collected by adjusting the reflux ratio to obtain 2620 grams, the rectification yield was 87%, and the gas chromatography content of the pure product was 99.6%.
Embodiment 13
[0105] Example 13 (preparation of 2-bromo-2,2-difluoroacetic acid)
[0106] Install a 3-liter three-necked flask with a reflux condenser, a mechanical stirring device, and a constant pressure dropping funnel. During the reaction, control the circulating temperature of the cooling water in the reflux condenser between 0 and 10°C, and connect the tail gas absorption device. Use sodium hydroxide aqueous solution to absorb the tail gas of the reaction, add 330 grams of water to the reaction bottle, control the reaction temperature between 0 and 20 °C, add 2901 grams of 2-bromo-2,2-difluoroacetyl chloride dropwise in 10 hours, drop After adding and keeping warm for 2 hours, change the experimental device into a rectifying device, install a rectifying column, a fractionating head-reflux condenser, and a magnetic stirring device that can adjust the reflux ratio on a 3-liter there-necked flask, and use an oil bath to heat the reaction bottle. The temperature of the oil bath is control...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com